Abstract
Background
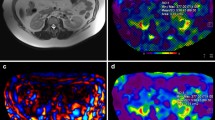
Renal parenchymal fibrosis is the most important determinant of kidney disease progression and it is determined via biopsy. The aim of this study is to evaluate the renal stiffness noninvasively by magnetic resonance elastography (MRE) and to compare it with clinicopathologic parameters in glomerulonephritis and AA amyloidosis patients.
Methods
Thirty-four patients with glomerular filtration rate (GFR) over 20 ml/min/1.73m2 had non-contrast MRE prospectively. Kidney stiffness values were obtained from whole kidney, cortex, and medulla. Values were correlated with GFR, albuminuria, proteinuria, and degree of fibrosis that are assessed via renal biopsy. Patients were grouped clinicopathologically to assess the relation between stiffness and chronicity.
Results
Mean whole kidney, cortex, and medulla stiffnesses were 3.78 (± 1.26), 3.63 (± 1.25), and 4.77 (± 2.03) kPa, respectively. Mean global glomerulosclerosis was 22% (± 18%) and median segmental glomerulosclerosis was 4% (min–max: 0%–100%). Extent of tubulointerstitial fibrosis was less than 25% in 26 of the patients (76.5%), 25%–50% in 6 of the patients (17.6%), and higher than 50% in 2 of the patients (5.9%). Fourteen patients were defined to have chronic renal parenchymal injury. MRE-derived stiffness values correlated negatively with parameters of fibrosis. Lower stiffness values were observed in patients with chronic renal injury compared to those without (P < 0.05 for whole kidney and medulla MRE-derived stiffness).
Conclusion
MRE-derived stiffness values were lower in patients with chronic injury. Stiffness decreases as glomerulosclerosis and tubulointerstitial fibrosis progresses in patients with primary glomerulonephritis and AA amyloidosis. With future studies, there may be a role for MRE to assess renal function in concert with conventional markers.







Similar content being viewed by others
References
Boor P, Ostendorf T, Floege J. Renal fibrosis: novel insights into mechanisms and therapeutic targets. Nat Rev Nephrol. 2010 Nov;6(11):643–56.
Rockey DC, Bell PD, Hill JA. Fibrosis--a common pathway to organ injury and failure. N Engl J Med. 2015 Mar;372(12):1138–49.
Roberts ISD, Cook HT, Troyanov S, Alpers CE, Amore A, Barratt J, et al. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009 Sep;76(5):546–56.
Risdon RA, Sloper JC, De Wardener HE. Relationship between renal function and histological changes found in renal-biopsy specimens from patients with persistent glomerular nephritis. Lancet (London, England). 1968 Aug;2(7564):363–6.
Brown RS, Sun MRM, Stillman IE, Russell TL, Rosas SE, Wei JL. The utility of magnetic resonance imaging for noninvasive evaluation of diabetic nephropathy. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 2020 Jun;35(6):970–8.
Luciano RL, Moeckel GW. Update on the Native Kidney Biopsy: Core Curriculum 2019. Am J kidney Dis Off J Natl Kidney Found. 2019 Mar;73(3):404–15.
Lees JS, McQuarrie EP, Mordi N, Geddes CC, Fox JG, Mackinnon B. Risk factors for bleeding complications after nephrologist-performed native renal biopsy. Clin Kidney J. 2017 Aug;10(4):573–7.
Roccatello D, Sciascia S, Rossi D, Naretto C, Bazzan M, Solfietti L, et al. Outpatient percutaneous native renal biopsy: safety profile in a large monocentric cohort. BMJ Open. 2017 Jun;7(6):e015243.
Fiorentino M, Bolignano D, Tesar V, Pisano A, Van Biesen W, D’’Arrigo G, et al. Renal Biopsy in 2015 - From Epidemiology to Evidence-Based Indications. Am J Nephrol [Internet]. 2016;43(1):1–19. Available from: https://www.karger.com/DOI/https://doi.org/10.1159/000444026
Jiang K, Ferguson CM, Lerman LO. Noninvasive assessment of renal fibrosis by magnetic resonance imaging and ultrasound techniques. Transl Res. 2019 Jul;209:105–20.
Kaimori J-Y, Isaka Y, Hatanaka M, Yamamoto S, Ichimaru N, Fujikawa A, et al. Visualization of kidney fibrosis in diabetic nephropathy by long diffusion tensor imaging MRI with spin-echo sequence. Sci Rep. 2017 Jul;7(1):5731.
Hueper K, Khalifa AA, Bräsen JH, Vo Chieu VD, Gutberlet M, Wintterle S, et al. Diffusion-Weighted imaging and diffusion tensor imaging detect delayed graft function and correlate with allograft fibrosis in patients early after kidney transplantation. J Magn Reson Imaging. 2016 Jul;44(1):112–21.
Xu X, Palmer SL, Lin X, Li W, Chen K, Yan F, et al. Diffusion-weighted imaging and pathology of chronic kidney disease: initial study. Abdom Radiol (New York). 2018 Jul;43(7):1749–55.
Feng Q, Ma Z, Wu J, Fang W. DTI for the assessment of disease stage in patients with glomerulonephritis--correlation with renal histology. Eur Radiol. 2015 Jan;25(1):92–8.
Friedli I, Crowe LA, Berchtold L, Moll S, Hadaya K, de Perrot T, et al. New Magnetic Resonance Imaging Index for Renal Fibrosis Assessment: A Comparison between Diffusion-Weighted Imaging and T1 Mapping with Histological Validation. Sci Rep. 2016 Jul;6:30088.
Zhao J, Wang ZJ, Liu M, Zhu J, Zhang X, Zhang T, et al. Assessment of renal fibrosis in chronic kidney disease using diffusion-weighted MRI. Clin Radiol. 2014 Nov;69(11):1117–22.
Leung G, Kirpalani A, Szeto SG, Deeb M, Foltz W, Simmons CA, et al. Could MRI Be Used To Image Kidney Fibrosis? A Review of Recent Advances and Remaining Barriers. Clin J Am Soc Nephrol. 2017 Jun;12(6):1019–28.
Kline TL, Edwards ME, Garg I, Irazabal M V, Korfiatis P, Harris PC, et al. Quantitative MRI of kidneys in renal disease. Abdom Radiol (New York). 2018 Mar;43(3):629–38.
Thiravit S, Suwanchatree P, Skulratanasak P, Thiravit P, Suvannarerg V. Correlation Between Apparent Diffusion Coefficient Values of the Renal Parenchyma and Estimated Glomerular Filtration Rates on 3-T Diffusion-Weighted Echo-Planar Magnetic Resonance Imaging. J Comput Assist Tomogr. 2019;43(5):780–5.
Toya R, Naganawa S, Kawai H, Ikeda M. Correlation between estimated glomerular filtration rate (eGFR) and apparent diffusion coefficient (ADC) values of the kidneys. Magn Reson Med Sci MRMS an Off J Japan Soc Magn Reson Med. 2010;9(2):59–64.
Namimoto T, Yamashita Y, Mitsuzaki K, Nakayama Y, Tang Y, Takahashi M. Measurement of the apparent diffusion coefficient in diffuse renal disease by diffusion-weighted echo-planar MR imaging. J Magn Reson Imaging. 1999 Jun;9(6):832–7.
Lee CU, Glockner JF, Glaser KJ, Yin M, Chen J, Kawashima A, et al. MR elastography in renal transplant patients and correlation with renal allograft biopsy: a feasibility study. Acad Radiol. 2012 Jul;19(7):834–41.
Marticorena Garcia SR, Fischer T, Dürr M, Gültekin E, Braun J, Sack I, et al. Multifrequency Magnetic Resonance Elastography for the Assessment of Renal Allograft Function. Invest Radiol. 2016 Sep;51(9):591–5.
Kim JK, Yuen DA, Leung G, Jothy S, Zaltzman J, Ramesh Prasad G V, et al. Role of Magnetic Resonance Elastography as a Noninvasive Measurement Tool of Fibrosis in a Renal Allograft: A Case Report. Transplant Proc. 2017 Sep;49(7):1555–9.
Kirpalani A, Hashim E, Leung G, Kim JK, Krizova A, Jothy S, et al. Magnetic Resonance Elastography to Assess Fibrosis in Kidney Allografts. Clin J Am Soc Nephrol. 2017 Oct;12(10):1671–9.
Samir AE, Allegretti AS, Zhu Q, Dhyani M, Anvari A, Sullivan DA, et al. Shear wave elastography in chronic kidney disease : a pilot experience in native kidneys. 2015;1–9.
Chen J, Kawashima A, Kim B, Kremers WK, Ehman RL, Gloor JM. MR Elastography in Renal Transplant Patients and Correlation with Renal Allograft Biopsy : A Feasibility Study. Acad Radiol [Internet]. 19(7):834–41. Available from: http://dx.doi.org/https://doi.org/10.1016/j.acra.2012.03.003
Toguchi M, Tsurusaki M, Yada N, Sofue K, Hyodo T, Onoda M, et al. Magnetic resonance elastography in the assessment of hepatic fibrosis: a study comparing transient elastography and histological data in the same patients. Abdom Radiol (New York). 2017 Jun;42(6):1659–66.
Han JH, Ahn J-H, Kim J-S. Magnetic resonance elastography for evaluation of renal parenchyma in chronic kidney disease: a pilot study. Radiol Med. 2020 May;
Schiller AM, Pellegrino PR, Zucker IH. Eppur Si Muove: The dynamic nature of physiological control of renal blood flow by the renal sympathetic nerves. Auton Neurosci. 2017 May;204:17–24.
Liu R, Das B, Xiao W, Li Z, Li H, Lee K, et al. A Novel Inhibitor of Homeodomain Interacting Protein Kinase 2 Mitigates Kidney Fibrosis through Inhibition of the TGF-β1/Smad3 Pathway. J Am Soc Nephrol. 2017 Jul;28(7):2133–43.
Karihaloo A. Anti-fibrosis therapy and diabetic nephropathy. Curr Diab Rep. 2012 Aug;12(4):414–22.
Acknowledgements
This study was supported by hacettepe üniversitesi with Grant No. TTU 2019-17871.
Author information
Authors and Affiliations
Contributions
İ.S.İ, A.S., M.K., and M.A. designed the study, A.T.G., C.C., C.Ö. M.Ü.K. T.Y., R.Y., B.A, and Y.E collected the data, A.T.G., İ.S.İ, A.S., M.K., and M.A. analyzed the data; A.T.G., İ.S.İ, A.S., M.K., and M.A. wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Güven, A.T., Idilman, I.S., Cebrayilov, C. et al. Evaluation of renal fibrosis in various causes of glomerulonephritis by MR elastography: a clinicopathologic comparative analysis. Abdom Radiol 47, 288–296 (2022). https://doi.org/10.1007/s00261-021-03296-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03296-1