Abstract
Purpose
To investigate the computed tomography (CT) and magnetic resonance imaging (MRI) characteristics of ovarian serous borderline tumors (SBTs), and evaluate whether CT and MRI can be used to distinguish micropapillary from typical subtypes.
Materials and methods
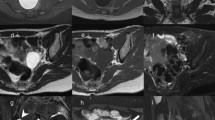
We retrospectively reviewed the clinical features and CT and MR imaging findings of 47 patients with SBTs encountered at our institute from September 2013 to December 2019. 30 patients with 58 histologically proven typical SBT and 17 patients with 26 micropapillary SBT were reviewed. Preoperative CT and MR images were evaluated, by two observers in consensus for the laterality, maximum diameter (MD), morphology patterns, internal architecture, attenuation or signal intensity, ADC value, enhancement patterns of solid portions (SP), and extra-ovarian imaging features.
Results
The median age were similar between typical SBT and SBT-MP (32.5 years, 36 years, respectively, P>0.05). Morphology patterns between two subtypes were significantly different on CT and MR images (P < 0.001). Irregular solid tumor (21/37, 56.76%) was the major morphology pattern of typical SBT tumor, while unilocular cyst with mural nodules (14/20, 70%) was the major morphology pattern of SBT-MP on CT images. Similarly, papillary architecture with internal branching (PA&IB) (17/21, 80.95%) was the major morphology pattern of typical SBT tumor, while unilocular cyst with mural nodules (4/6, 66.67%) was the major pattern of SBT-MP on MR images. PA&IB all showed slightly hyperintense papillary architecture with hypointense internal branching on T2-weighted MRI. More calcifications were found in typical SBT (24/37, 64.86%) than SBT-MP mass lesion (6/20, 30%) (P < 0.05). Hemorrhage was less frequently visible in (20/37, 54.05%) typical SBT lessons than SBT-MP mass lesion (18/20, 90%) (P < 0.05). The ovarian preservation is more seen in typical SBT (38/58, 65.52%) than SBT-MP (12/28, 42.86%) in our series (P < 0.05). Mean ADC value of solid portions (papillary architecture and mural nodules) was 1.68 (range from 1.44 to 1.85) × 10−3 mm2/s for typical SBT and 1.62 (range from 1.45 to 1.7) × 10−3 mm2/s for that of SBT-MP. The solid components of the two SBT subtypes showed wash-in appearance enhancements after contrast injection both in CT and MR images except 2 of SBT-MP with no enhancement as complete focal hemorrhage on MR images.
Conclusion
Morphology and internal architecture are two major imaging features that can help to distinguish between SBT-MP and typical SBT.




Similar content being viewed by others
References
Kurman R, et al. WHO classification of Tumours of female reproductive organs. No 6. IARC WHO classification of Tumours, fourth edition. IARC: Lyon; 2014.
May T, Virtanen C, Sharma M, et al. Low malignant potential tumors with micropapillary features. Gynecol Oncol 2010;117(1):9-17.
Burks RT, Sherman ME, Kurman RJ. Micropapillary serous carcinoma of the ovary. A distinctive low-grade carcinoma related to serous borderline tumors. Am J Surg Pathol 1996; 20(11):1319-1330.
Seidman JD, Kurman RJ. Subclassification of serous borderline tumors of the ovary into benign and malignant types. A clinicopathologic study of 65 advanced stage cases. Am J Surg Pathol 1996; 20(11):1331-1345.
Wei wei, Guo qing, Tu ping, et al. Clinicopathological features of ovarian serous borderline tumor. J Clin Exp Pathol 2013;29(6):615-620.
Nakai G, Yamada T, Yamamoto K, et al. MRI appearance of ovarian serous borderline tumors of the micropapillary type compared to that of typical ovarian serous borderline tumors: radiologic-pathologic correlation. J Ovarian Res 2018;11(1):7.
Wong HF, Low J, Chua Y, et al. Ovarian tumors of borderline malignancy: a review of 247 patients from 1991 to 2004. Int J Gynecol Cancer 2007;17(2):342-349.
Tempfer CB, Polterauer S, Bentz EK, et al. Accuracy of intraoperative frozen section analysis in borderline tumors of the ovary: a retrospective analysis of 96 cases and review of the literature. Gynecol Oncol 2007;107:248e52.
Morotti M, Menada MV, Gillott DJ, et al. The preoperative diagnosis of borderline ovarian tumors: a review of current literature. Arch Gynecol Obstet 2011; 285(4):1103-1112.
Makar AP, Kaern J, Kristensen GB, et al. Evaluation of serum CA125 level as a tumor marker in borderline tumors of the ovary. Int J Gynecol Cancer 1993; 3(5):299-303.
Candido Dos Reis F, Moreira de Andrade J, Bighetti S. CA 125 and vascular endothelial growth factor in the differential diagnosis of epithelial ovarian tumors. Gynecol Obstet Invest 2002; 54(3):132-136.
Kim SH, Yang DM, Kim SH. Borderline serous surface papillary tumor of the ovary: MRI characteristics. AJR Am J Roentgenol 2005;184(6):1898-1900.
Tanaka YO, Okada S, Satoh T, et al. Ovarian serous surface papillary borderline tumors form sea anemone-like masses. J Magn Reson Imaging 2011;33(3):633-640.
Shadbolt C, Kouloyan-Ilic S, Dobrotwir A. Borderline ovarian tumours: MRI features to aid a challenging diagnosis. In: The royal Australian and New Zealand college of radiologists annual scientific meeting; 2013.
Naqvi J, Nagaraju E, Ahmad S. MRI appearances of pure epithelial papillary serous borderline ovarian tumours. Clinical Radiol 2015;70(4): 424-432.
Outwater EK, Huang AB, Dunton CJ, et al. Papillary projections in ovarian neoplasms: appearance on MRI. J Magn Reson Imaging 1997;7(4):689-695.
Dietel M, Hauptmann S. Serous tumors of low malignant potential of the ovary. 1. Diagnostic pathology. Virchows Arch 2000; 436(5):403-412.
Zhao SH, Qiang JW, Zhang GF, et al. Diffusion-weighted MR imaging for differentiating borderline from malignant epithelial tumours of the ovary: pathological correlation. Eur Radiol. 2014; 24(9):2292-2299.
Stevens SK, Hricak H, Stern JL. Ovarian lesions: detection and characterization with gadolinium-enhanced MR imaging at 1.5 T. Radiology 1991;181(2):481-488.
Woodward PJ, Hosseinzadeh K, Saenger JS. From the archives of the AFIP: radiologic staging of ovarian carcinoma with pathologic correlation. Radiographics 2004;24(1):225-246.
Kawamoto S, Urban BA, Fishman EK. CT of epithelial ovarian tumors. Radiographics 1999; 19:S85-S102.
Acs G. Serous and mucinous borderline (low malignant potential) tumors of the ovary. Am J Clin Pathol 2005;123: S13-S57.
Jung SE, Lee JM, Rha SE, et al. CT and MR imaging of ovarian tumors with emphasis on differential diagnosis. Radiographics 2002;22(6):1305-1325.
Thomassin-Naggara I, Daraï E, Cuenod CA, et al. Dynamic contrastenhanced magnetic resonance imaging: a useful tool for characterizing ovarian epithelial tumors. J Magn Reson Imaging 2008;28(1):111-120.
Bazot M, Haouy D, Daraï E, et al. Is MRI a useful tool to distinguish between serous and mucinous borderline ovarian tumours? Clin Radiol 2013;68(1):e1-8.
Burkholz KJ, Wood BP, Zuppan C. Best cases from the AFIP: Borderline papillary serous tumor of the right ovary. Radiographics 2005;25:1689–1692.
Malpica A, Deavers MT, Lu K, et al. Grading ovarian serous carcinoma using a two-tier system. Am J Surg Pathol. 2004; 28(4):496-504.
Zhao SH, Qiang JW, Zhang GF, et al. MRI appearances of ovarian serous borderline tumor: pathological correlation. J Magn Reson Imaging 2014;40:151-156.
Van Vierzen PB, Massuger LF, Ruys SH, et al. Borderline ovarian malignancy: ultrasound and fast dynamic MR findings. Eur J Radiol 1998;28(2):136-142.
Tavassoli FA, Devilee P. International agency for research on cancer. Pathology and genetics of tumours of the breast and female genital organs. Geneva: World Health Organization; 2003.
Bent CL, Sahdev A, Rockall AG, et al. MRI appearances of borderline ovarian tumours. Clin Radiol 2009;64(4):430-438.
Okamoto Y, Tanaka YO, Tsunoda H, et al. Malignant or borderline mucinous cystic neoplasms have a larger number of loculi than mucinous cystadenoma: a retrospective study with MR. J Magn Reson Imaging 2007;26(1):94-99.
Nougaret S, Lakhman Y, Molinari N, et al. CT Features of Ovarian Tumors: Defining Key Differences Between Serous Borderline Tumors and Low-Grade Serous Carcinomas. AJR Am J Roentgenol 2018;210(4), 918-926.
Okada S, Ohaki Y, Inoue K, et al. Calcifications in Mucinous and Serous Cystic Ovarian Tumors. J Nippon Med Sch 2005; 72(1):29-33.
Dobson M, Carrington BM, Radford JA, et al. The role of computed tomography in the management of ovarian tumours of borderline malignancy. Clin Radiol 1997;52(4):280-283.
Sherman ME, Mink PJ, Curtis R, et al. Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis. Cancer 2004;100(5):1045-1052.
Funding
This study has received funding from Yunnan Applied Basic Research Projects-Joint Special Project (Grant Number 2019FE001-246).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest and complied with ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhang, Y., Tan, J., Wang, J. et al. Are CT and MRI useful tools to distinguish between micropapillary type and typical type of ovarian serous borderline tumors?. Abdom Radiol 46, 3354–3364 (2021). https://doi.org/10.1007/s00261-021-03000-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-021-03000-3