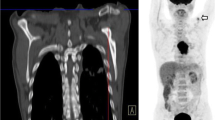
Abstract
Objective
The aim of this study was to evaluate PET/FDG metabolic parameters in locally advanced GEJC and correlate it with molecular pathological profiles.
Methods
We retrospectively analyzed data from 66 patients with a histopathological diagnosis of GEJC who had undergone 18F-FDG PET/CT before surgical resection. Maximum standardized uptake (SUVmax), mean standardized uptake (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG) of the primary tumor were measured and calculated using the region of interest (ROI) technique. The relationship between metabolic parameters and the Lauren’s classification, histologic differentiation, Ki-67 staining and positivity for human epidermal growth factor receptor 2 (HER2), c-Met, and epidermal growth factor receptor (EGFR) were investigated through immunohistochemical (IHC) analyses.
Results
Of the total 66 patients, significant differences were observed between intestinal and non-intestinal (mixed and diffuse) adenocarcinomas in SUVmax (8.23 ± 2.83 vs. 6.29 ± 2.41, P = 0.008), SUVmean (4.85 ± 1.47 vs. 3.93 ± 1.22, P = 0.017), MTV (24.96 cm3 vs. 8.90 cm3; P = 0.004), and TLG (97.38 cm3 vs. 37.09 cm3, P = 0.005) values. SUVmax, MTV, and TLG of moderately differentiated adenocarcinomas were significantly higher than those of the poorly differentiated ones. SUVmax was significantly higher in tissues with a higher Ki-67 index or in the c-MET-negative group (P = 0.045, P = 0.036). No significant correlation was found between metabolic parameters and the expression of HER2 or EGFR in GEJC.
Conclusion
18F-FDG PET/CT may be useful for predicting the molecular pathological profiles of GEJC and for determining appropriate therapeutic strategy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The incidence of gastroesophageal junction carcinoma (GEJC) has dramatically increased over the last few decades and it is currently a major cause of cancer-related morbidity and mortality worldwide [1, 2]. Although there has been progress in the development of cytotoxic chemotherapy for GEJC, the outcomes remain poor, especially in advanced disease, as the treatment strategy and prognosis depend on multiple factors, including histological grade, stage of the disease, presence and extent of lymph node metastasis, and extent of lymph node dissection [3, 4].
Recently, a genomic analysis of GEJC, reported in the Cancer Genome Atlas (CGA), has revealed that the molecular features and candidate pathways of GEJC differ from that of oesophageal carcinoma (EC) and gastric carcinoma (GC). Specifically, recurrent co-amplification of members of the receptor tyrosine kinase (RTK) family, such as epidermal growth factor receptor (EGFR), MET, and cell cycle regulators like the human epidermal growth factor receptor 2 (HER2) have been described [5, 6], and overexpression of these markers has been reported to be associated with pharmaceutical development and poor prognosis in GEJC [7,8,9]. Thus, characterizing GEJC into subtypes based on histology and genotype will provide a roadmap for tumor characterisation, patient stratification, and thereby, personalized medicine.
Histological and immunohistochemical (IHC) analysis are the standard methods used to define the pathological features and mutation marker expression in GEJC, but these procedures are both invasive and postoperative. Thus, alternate non-invasive strategies for predicting these profiles, such as 18F-FDG PET/CT, offer great value [10]. This multimodality imaging system co-registers metabolic and morphologic data and is recognized as a useful diagnostic technique in many malignant tumors, including GEJC. Lee et al. [11] have reported that 18F-FDG PET/CT has a high detection rate in advanced gastric cancer, including metastases. Kim et al. have demonstrated that diffuse-type adenocarcinoma, classified according to the Lauren classification, has a low maximal standardized uptake value (SUVmax) in patients with locally advanced GC [3]. Jia et al. have reported that PET/CT can potentially predict tumor phenotype, such as its MET or EGFR mutation status, in GEJC and gastric cancer [12]. However, some recent studies have shown inconsistent results while using the 18F-FDG PET/CT to evaluate recurrent GEJC and GC, and there are concerns that its low diagnostic sensitivity may limit its usefulness.
Recent investigations on metabolic tumor volume (MTV) and total lesion glycolysis (TLG), which represent tumor burden, have demonstrated a significantly higher predictive value for these two parameters compared to SUVmax [13,14,15,16]. However, these studies have provided limited data on whether metabolic parameters of GEJC lesions are related to histopathologic features and molecular markers.
Thus, the aim of this study was to retrospectively analyze data from GEJC patients who had undergone tumor staging by 18F-FDG PET/CT at our institution to evaluate any potential relationship between preoperative pathological features and molecular marker expression associated with higher values of select metabolic parameters such as SUVmax, SUVmean, MTV, and TLG. Significant predictors of 18F-FDG-avid lesions were used to stratify GEJC patients after lesion detection and for making treatment decisions.
Materials and methods
Patients
This study was performed after approval by the Investigational Review Board of the Peking University Cancer Hospital. The requirement to obtain informed consent was waived due to the Ethics Committee of Peking University Cancer Hospital. Data from consecutive patients evaluated between January 2010 and December 2015 at the Beijing Cancer Hospital were retrospectively screened from Electronic Medical Records System for inclusion according to the following criteria: (a) histologically proven GEJC; (b) no prior local treatment or systematic chemotherapy; (c) pretreatment 18F-FDG PET/CT scan; (d) should have undergone a radical total or subtotal gastrectomy; (e) the presence of complete medical history and clinicopathological data. The exclusion criteria were (a) secondary malignant disease; (b) serious infection or inflammation (e.g., HIV); or (c) hepatic or renal dysfunction.
18F-FDG PET/CT
Preoperative 18F-FDG PET/CT scans and intravenous injection dose were performed by technologist and nurse in Department of Nuclear Medicine of Peking University Cancer Hospital. The time interval between PET/CT examination and surgery is 30 (2–223). Patients were instructed to fast for at least 6 h before the scan to ensure serum glucose levels of < 10 mmol/L. 18F-FDG was intravenously administered at a dose of 3.7 MBq/kg. At approximately 60 ± 10 min post-injection, a whole-body acquisition was performed in 6–8 bed positions (1 min/bed) using a hybrid system (PHILIPS Gemini TF) that covered the area from the base of the skull to the upper thigh. A non-contrast-enhanced CT was acquired using the following settings: modulated 100 mAs, 120 kV, and slice thickness of 3 mm for attenuation correction and anatomical localization purposes. Head image acquisition was performed in one bed position (8–10 min/bed). For reconstruction, attenuation-weighted ordered-subsets expectation maximization (AW-OSEM) iterative algorithm with 4 iterations and 8 subsets, Gaussian filter with 4.0 mm full width at half maximum (FWHM), and scatter correction were used. Attenuation correction was based on the portal venous phase of the whole-body CT scan.
Quantitative PET parameter computation
Two physicians experienced in nuclear medicine were assigned to blindly and independently interpret each patient’s PET images and data at a PHILIPS EBW workstation. The following parameters were calculated in 3D mode using vendor-provided software, namely, maximum standard uptake volume (SUVmax), mean SUV (SUVmean), metabolic tumor volume (MTV), and total lesion glycolysis (TLG). MTV was estimated using the adaptive threshold method [17] for each primary gastric lesion, by selecting a volume of interest (VOI) on the axial image, and the size of VOI was verified on the corresponding coronal and sagittal images to include the entire tumor in the VOI. TLG was calculated as the product of SUVmean and MTV.
Histopathologic classification
All GEJC diagnoses were provided by specialty-trained gastrointestinal pathologists. Sections of 4 µM thickness were cut from wax blocks, mounted on to poly-l-lysine coated microscope slides, sections stained with haematoxylin and eosin, and used for histopathologic determinations, such as tumor category, histological grade, Lauren classification, and the presence of lymph node metastasis.
Immunohistochemistry
Immunohistochemistry was performed using the PowerVision Two-Step Histostaining Reagent (ImmunoVision Technologies, Brisbane, CA). Briefly, formalin-fixed, paraffin-embedded blocks were cut into 4-μm-thick sections, deparaffinized in xylene and rehydrated. Antigen retrieval was performed using EDTA (pH 8.0; Santa Cruz Biochemistry, Dallas, TX) in a pressure cooker for 3 min. The sections were the incubated in 3% H2O2 solution for 10 min at room temperature to block endogenous peroxidase activity.
Each section was assessed via semiquantitative analysis. HER2 immunohistochemical staining was performed for all tumors using anti-HER2 rabbit polyclonal antibodies (clone 4B5, Ventana, Rotkreuz, Switzerland) on a Ventana Benchmark XT automated staining platform with an iView 3,3′-diaminobenzidine tetrahydrochloride (3,3′-diaminobenzidine) detection kit (Ventana), according to manufacturer’s instructions. A previously described scoring system was used [18]. Briefly, cases with < 10% discernible membrane staining or totally negative staining were scored as 0 + ; cases with weak and/or incomplete basolateral or membrane staining requiring magnification of at least 200 × to confirm positivity were scored as 1 + ; cases with weak to moderate, continuous basolateral, or membrane staining that was not readily apparent at low (40 ×) magnification were scored as 2 + ;and cases with strong basolateral or membranous staining that could easily be detected at low magnification were scored as 3 + . HER2 was considered positive for IHC scores of 2 + or 3 + .
Immunohistochemical staining of Ki-67 was conducted at the Peking University Cancer Hospital. It was automatically performed using a primary antibody against Ki-67 (Clone MIB-1; DAKO, USA); the staining intensity was scored as 1 if < 75% of tumor cells were stained and 2 if ≥ 75% of tumor cells were stained. A score of 1 was interpreted as Ki-67-low, whereas scores of 2 were considered Ki-67-high.
EGFR and c-MET were automatically stained using the CONFIRM anti-EGFR (EP38Y) primary antibody (Dako EGFR pharmDx kit) and the CONFIRM anti-total c-MET (SP45) rabbit monoclonal primary antibody (Ventana Medical Systems). Membrane staining intensity in terms of EGFR was scored as previously described [19]. Briefly, cases of no reactivity or membranous reactivity in < 10% of the tumor cells were scored as 0; cases of faint/barely perceptible membranous reactivity in at least 10% of the tumor cells or cells with partly reactive membranes were scored as 1 + ; cases presenting weak to moderate complete or basolateral membranous reactivity in at least 10% of the tumor cells were scored as 2 + ; and cases presenting moderate to strong complete or basolateral membranous reactivity in at least 10% of the tumor cells were scored as 3 + . EGFR was considered positive if the IHC score was 2 + or 3 + . Membrane staining intensity in terms of c-MET was evaluated as previously reported [20]. Briefly, staining patterns were scored as follows: cases presenting no or some reactivity in < 50% of the tumor cells were scored as 0; cases presenting weak or higher membranous reactivity in at least 50% of the tumor cells but moderate or higher membranous reactivity in < 50% were scored as 1 + ; cases presenting moderate or higher membranous reactivity in at least 50% of the tumor cells but strong membranous reactivity in < 50% of the tumor cells were scored as 2 + ; and cases presenting strong membranous reactivity in at least 50% of the tumor cells were scored as 3 + . An intensity score of 2 + or 3 + was considered positive expression. All slides were independently evaluated and scored by two pathologists who were blinded to the patient data. Cases with disputed scores were re-examined under a discussion microscope.
Statistical analysis
Statistical analysis was performed using SPSS software 23.0, (Inc.). Data are presented as mean ± SD or median (Q1–Q3), depending on normality of distribution. The independent t test, one-way ANOVA, and Pearson’s Chi-square tests were used to evaluate significant differences between continuous and categorical variables, respectively. Correlation between variables was computed using the non-parametric Spearman correlation test. Receiver operating characteristic (ROC) curves were used to define optimal cut-off values for SUVmax, and sensitivity and specificity of the predictive ability of Ki-67 and c-Met expression. The threshold for statistical significance was set at P = 0.05.
Results
Patient characteristics
Records of 812 patients were screened, among which 66 cases constituted the study dataset (Fig. 1).
The clinical characteristics of the 66 patients whose data were analyzed in this study are summarized in Table 1. The median age of the patients was 64 years (range 48–80 years), and 59 of these patients (89%) were men. Thirty-one patients (47%) had intestinal type lesions, while 35 (53%) cases were non-intestinal; 43% of adenocarcinomas were moderately differentiated, 41% were moderately poorly differentiated, and 16% were poorly differentiated. Lymph node metastasis was detected in 42 cases.
The SUVmax and SUVmean for the primary lesion were 7.19 ± 2.69 and 4.35 ± 1.36, respectively. The median MTV and TLG values were 13.95 ncm3 (range 2.18–104.26) and 63.39 cm3 (range 7.53–502.51), respectively. No significant differences among metabolic parameters were found when analyzed according to gender, age, T category, or lymph node metastasis.
Histopathological profiles
Significant differences in SUVmax (8.23 ± 2.83 vs. 6.29 ± 2.41, P = 0.008), SUVmean (4.85 ± 1.47 vs. 3.93 ± 1.22, P = 0.017), MTV (24.96 cm3 vs. 11.17 cm3; P = 0.004), and TLG (97.38 cm3 vs. 47.12 cm3, P = 0.005) were observed between intestinal and non-intestinal adenocarcinoma (Fig. 2). Correlation analysis revealed a mild correlation between Lauren’s classification and the four metabolic parameters analyzed (P < 0.05 for all), namely, SUVmax, SUVmean, TLG, and MTV (r = 0.27).
Moderately differentiated adenocarcinomas exhibited significantly higher SUVmax (8.46 ± 2.81), MTV (24.96 cm3), and TLG (97.38 cm3) values than did moderately poorly (6.80 ± 2.48, 10.08 cm3, and 28.46 cm3, respectively) or poorly differentiated adenocarcinomas (5.85 ± 2.27, 8.77 cm3, and 28.46 cm3, respectively; P values of 0.044, 0.001, and 0.001, separately). Correlation analysis showed that tumor differentiation was closely related to SUVmax, MTV, and TLG values with ‘r’ values of 0.33, 0.47, and 0.47 (Fig. 3). No correlation was found between SUVmean and tumor differentiation (P = 0.066). Further, intestinal adenocarcinoma that were moderately differentiated had significantly higher SUVmax, MTV, and TLG values than non-intestinal and moderately poorly or poorly differentiated tumors (P = 0.046, P = 0.001, and P = 0.001, respectively; Table 2).
Ki-67 index
Patients were categorized into 2 groups according to IHC staining for Ki-67, i.e., Ki-67 expression ≥ 75% (n = 22) and Ki-67 expression < 75% (n = 30). There was a significant difference in SUVmax between GEJC with higher Ki-67 index and lower Ki-67 index (6.97 ± 2.15 vs. 8.49 ± 3.20; P = 0.045; Table 1, Fig. 4), while there were no significant differences in SUVmean, MTV, and TLG between these two groups. ROC analysis showed that the optimal cut-off value for adequate sensitivity and specificity of SUVmax as a predictor for Ki-67 expression was 7.27 (sensitivity 68.2% and specificity 70.0%). Lesions with SUVmax greater than this optimal threshold might have higher Ki-67 expression.
Molecular tumor markers
c-MET overexpression was observed in 54 patients; of these 7 (13.0%) were scored as c-MET-positive and 47 (87.0%) as negative. The expression of c-MET was significantly correlated with SUVmax (P = 0.036), but not with SUVmean, MTV, or TLG values (P = 0.490, 0.361, and 0.321, respectively, Table 1, Fig. 4). c-MET-negative GEJC had a higher SUVmax value (6.43 ± 1.03) than c-MET-positive cases (7.69 ± 2.84). The threshold value of SUVmax as a predictor of c-MET expression was 7.01 with a sensitivity of 85.7% and a specificity of 61.7%.
EGFR analysis was performed in samples from 54 patients; of these, 22 were EGFR+ and 32 were EGFR− . No correlation was found between EGFR expression and metabolic parameters. However, it was observed that c-MET expression correlated with EGFR expression in GEJC patients (P = 0.011, Fig. 5).
HER2 overexpression was analyzed in 56 patients (for there were 2 patients who have only experienced HER2 test but not c-MET or EGFR) with GEJC, and 24 were HER2+ while 32 were HER2− . No differences were seen between HER2+ and HER2− cases with respect to SUVmax (7.31 ± 3.03 vs. 7.60 ± 2.41, P = 0.693), SUVmean (4.51 ± 1.66 vs. 3.93 ± 1.22, P = 0.857), MTV (13.54 cm3 vs. 16.10 cm3; P = 0.728), or TLG (63.93 cm3 vs. 77.55 cm3, P = 0.741) values. However, a correlation between Ki-67 index and HER2 expression was obtained in GEJC lesions alone (P = 0.047, Fig. 5).
Discussion
We show that tumor metabolism, measured by SUVmean, SUVmax, MTV, and TLG of 18F-FDG PET/CT, correlate with some pathological features and mutation marker expression in patients with GEJC. The strong predictive value of SUVmax could also be confirmed with the Ki-67 index and c-Met mutation status. However, we found no association between metabolic parameters and HER2 or EGFR expression status in GEJC.
Accurate preoperative staging is essential to determine the most effective treatment modality in patients with resectable GEJC lesion [21]. The current standards for staging of gastric cancer use CT, gastroscopy, and laparoscopy. The routine use of 18F-FDG PET/CT in preoperative staging is considered to be of limited value because of its varied sensitivity (21% to 100% [22, 23]) in detecting primary GEJC lesions and lymph node metastases, suggesting that multiple factors influence the magnitude of 18F-FDG uptake in GEJC lesions. This may be explained by the fact that GEJCs indeed comprise a heterogeneous group of tumors with dissimilar biological characteristics, which in turn can lead to divergent metabolic and bioenergetic properties. Thus, 18F-FDG PET/CT results may be useful in supporting clinical decision making, selecting patients who may benefit from staging, monitoring treatment response and prognosis, and conducting surveillance for recurrent disease.
Several studies have reported higher 18F-FDG uptake values in intestinal type lesions than diffuse-type lesions, as classified according to the Lauren classification [22, 24]. Low 18F-FDG uptake in some GEJC and GC may be explained by the higher extracellular mucin content and a greater dispersion and dilution of the tumor cells [25]. Here, we not only show that the Lauren intestinal type GEJC had higher SUVs (both SUVmean and SUVmax) than the non-intestinal type, but also that this type of GEJC displays higher MTV and TLG, which are volume-based parameters that also provide metabolic information on metastatic lesions, treatment response, and prognosis. Similar results were found in the correlation between primary tumor 18F-FDG uptake and its histologic grade. Specifically, we observed that moderately differentiated GEJC had higher metabolic parameters than poorly differentiated lesions, and that histologic grades were closely related to SUVmax, MTV, and TLG. We also stratified GEJC by both the Lauren classification and its histologic grade, and found that the intestinal type and moderately differentiated lesions were frequently 18F-FDG-avid rather than non-avid, whereas the reverse was true for non-intestinal poorly differentiated tumors.
It has been previously reported that the intestinal type or well-differentiated tumor with a high Ki-67 index, a cellular proliferation marker, is associated with a more favorable prognosis in patients with advanced GEJC and GC [26,27,28]. Even though many studies have demonstrated a correlation between SUVs and the Ki-67 index in many solid tumors, such as lung cancer [29], breast cancer [30], or oesophageal cancer [31], the predictive value of SUVs with respect to Ki-67 status in GEJC remains unclear. In this context, our results, which show an excellent correlation between Ki-67 index and SUVmax, can be used to predict Ki-67 expression in the primary GEJC lesion.
Further, identifying these 18F-FDG-avid predictors in GEJCs, as mentioned above, could enable the use of the 18F-FDG PET/CT with greater confidence for assessing the extent of disease before planned surgical resection or surveillance for recurrent disease.
At present, treatment decisions for GEJC are based on pathologic assessment of the resected tissue specimen, and perioperative systemic chemotherapy is used in most patients with locally advanced disease. Currently, targeted therapies are being increasingly incorporated into the therapeutic algorithm because of the availability of data from genomic analysis. Amplification of genes such as HER2, EGFR, and c-MET in GEJC makes them suitable molecular targets for targeted therapies and immunotherapy. Accurate evaluation of the expression status of these markers can help identify GEJC patients who will respond best to such targeted therapies. However, the diagnostic applicability of molecular markers remains challenging owing to considerable intra-tumoral heterogeneity of the GEJC, which can lead to sampling errors. The PET/CT is a molecular imaging technique widely used in the diagnosis and staging of malignant tumors, and the identification and validation of imaging biomarkers that can predict overexpression of HER2, EGFR, and c-MET in tumors would be a welcome tool, particularly if they can contribute toward making effective treatment decisions.
In clinical practice, HER2 was the first and only membrane-bound RTK to be successfully targeted in the treatment of patients with GC and GEJC [32]. Many studies have attempted to reveal the relationship between HER2 expression and FDG metabolism; however, the results remain controversial [10, 33]. Specifically, while some studies have reported that the SUVmax is significantly lower in HER2-positive group than in the HER2-negative group [10], others have drawn the opposite conclusion [33]. Consistent with results reported by Celli et al. [34], our data demonstrate that there is no significant difference in SUVmax between HER2 + and HER2− groups, implying that this marker cannot be used to predict the HER2 expression status of GEJC on initial staging.
Until recently, targeting the HGF/MET axis with monoclonal antibodies or small-molecule inhibitors has not led to compelling efficacy in patients with GEJC/GC. Alternative biomarkers, other than determining MET status by IHC, might be required to guide patient selection and for achieving meaningful use of these anti-HGF/MET treatments. In this study, we observed patients with SUVmax below 7.01 displayed a higher possibility of c-MET overexpression. This promising result suggests that SUVmax is a potential marker to select GEJC tumors that will respond to MET inhibitors. Unfortunately, metabolic parameters failed to predict EGFR expression in GEJC patients.
To the best of our knowledge, this is the first study that has investigated whether the tumor metabolic burden can predict the pathological profile and molecular markers in GEJC. However, our study was partly limited by its retrospective design and the small sample size.
Conclusion
The intestinal type and moderately differentiated GEJCs have higher SUVmax, MTV, and TLG than the non-intestinal and poorly differentiated types. SUVmax was significantly higher in lesions with a high Ki-67 index or those that are c-Met-negative. No significant correlation was found between metabolic parameters and the expression of HER2 and EGFR in GEJC. 18F-FDG PET/CT may be useful in predicting the pathological characteristics and molecular markers of GEJC, and for determining appropriate and effective therapeutic strategies. Lastly, large prospective studies are needed to confirm our results and determine whether metabolic imaging can indeed be used to determine the pathological profiles GEJC lesions.
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136:E359-86.
Dolan K, Sutton R, Walker SJ, et al. New classification of oesophageal and gastric carcinomas derived from changing patterns in epidemiology. Br J Cancer 1999; 80:834-42
Kim HW, Won KS, Song BI, Kang YN. Correlation of Primary Tumor FDG Uptake with Histopathologic Features of Advanced Gastric Cancer. Nucl Med Mol Imaging 2015; 49:135-42.
Guggenheim DE, Shah MA. Gastric cancer epidemiology and risk factors. J Surg Oncol 2013; 107:230-6.
Cancer Genome Atlas Research N, Analysis Working Group: Asan U, Agency BCC, et al. Integrated genomic characterization of oesophageal carcinoma. Nature 2017; 541:169-175.
Cancer Genome Atlas Research N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513:202-9.
Waddell T, Chau I, Cunningham D, et al. Epirubicin, oxaliplatin, and capecitabine with or without panitumumab for patients with previously untreated advanced oesophagogastric cancer (REAL3): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:481-9
Lordick F, Kang YK, Chung HC, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 2013; 14:490-9
Iveson T, Donehower RC, Davidenko I, et al. Rilotumumab in combination with epirubicin, cisplatin, and capecitabine as first-line treatment for gastric or oesophagogastric junction adenocarcinoma: an open-label, dose de-escalation phase 1b study and a double-blind, randomised phase 2 study. Lancet Oncol 2014; 15:1007-18
Chen R, Zhou X, Liu J, Huang G. Relationship Between 18F-FDG PET/CT Findings and HER2 Expression in Gastric Cancer. J Nucl Med 2016; 57:1040-4
Lee JW, Lee SM, Lee MS, Shin HC. Role of (1)(8)F-FDG PET/CT in the prediction of gastric cancer recurrence after curative surgical resection. Eur J Nucl Med Mol Imaging 2012; 39:1425-34
Jia YX, Li TF, Zhang DD, et al. The coexpression and prognostic significance of c-MET, fibroblast growth factor receptor 2, and human epidermal growth factor receptor 2 in resected gastric cancer: a retrospective study. Onco Targets Ther 2016; 9:5919-5929.
Kawada K, Toda K, Nakamoto Y, et al. Relationship Between 18F-FDG PET/CT Scans and KRAS Mutations in Metastatic Colorectal Cancer. J Nucl Med 2015; 56:1322-7
Hatt M, van Stiphout R, le Pogam A, et al. Early prediction of pathological response in locally advanced rectal cancer based on sequential 18F-FDG PET. Acta Oncol 2013; 52:619-26
Chen HH, Chiu NT, Su WC, et al. Prognostic value of whole-body total lesion glycolysis at pretreatment FDG PET/CT in non-small cell lung cancer. Radiology 2012; 264:559-66
Song MK, Chung JS, Lee JJ, et al. Metabolic tumor volume by positron emission tomography/computed tomography as a clinical parameter to determine therapeutic modality for early stage Hodgkin’s lymphoma. Cancer Sci 2013; 104:1656-61
Im HJ, Bradshaw T, Solaiyappan M, Cho SY. Current Methods to Define Metabolic Tumor Volume in Positron Emission Tomography: Which One is Better? Nucl Med Mol Imaging 2018; 52:5-15.
Radu OM, Foxwell T, Cieply K, et al. HER2 amplification in gastroesophageal adenocarcinoma: correlation of two antibodies using gastric cancer scoring criteria, H score, and digital image analysis with fluorescence in situ hybridization. Am J Clin Pathol 2012; 137:583-94
Nagatsuma AK, Aizawa M, Kuwata T, et al. Expression profiles of HER2, EGFR, MET and FGFR2 in a large cohort of patients with gastric adenocarcinoma. Gastric Cancer 2015; 18:227-38
Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013; 31:4105-14
Patru CL, Surlin V, Georgescu I, Patru E. Current issues in gastric cancer epidemiology. Rev Med Chir Soc Med Nat Iasi 2013; 117:199-204
Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003; 30:288-95
Kim EY, Lee WJ, Choi D, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol 2011; 79:183-8
Lorenzen S, Herrmann K, Weber WA, et al. Visualisation of metastatic oesophageal and gastric cancer and prediction of clinical response to palliative chemotherapy using 18FDG PET. Nuklearmedizin 2007; 46:263-70
Chung HW, Lee SY, Han HS, et al. Gastric cancers with microsatellite instability exhibit high fluorodeoxyglucose uptake on positron emission tomography. Gastric Cancer 2013; 16:185-92
Cimerman M, Repse S, Jelenc F, et al. Comparison of Lauren’s, Ming’s and WHO histological classifications of gastric cancer as a prognostic factor for operated patients. Int Surg 1994; 79:27-32
Ross W, Hall PA. Ki67: from antibody to molecule to understanding? Clin Mol Pathol 1995; 48:M113-7
Li S, Li J, Liu Z, et al. [Predictive value of P53, Ki-67, HER2 protein detection in neoadjuvant chemotherapy for adenocarcinoma of gastroesophageal junction]. Zhonghua Wei Chang Wai Ke Za Zhi 2015; 18:901-4.
Park S, Lee E, Rhee S, et al. Correlation between Semi-Quantitative (18)F-FDG PET/CT Parameters and Ki-67 Expression in Small Cell Lung Cancer. Nucl Med Mol Imaging 2016; 50:24-30
Koo HR, Park JS, Kang KW, et al. Correlation between (18)F-FDG uptake on PET/CT and prognostic factors in triple-negative breast cancer. Eur Radiol 2015; 25:3314-21
Mu DB, Wang SP, Yang WF, et al. [Correlation between FDG PET/CT and the expression of glutl and ki-67 antigen in esophageal cancer]. Zhonghua Zhong Liu Za Zhi 2007; 29:30-3.
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376:687-97
Kim JS, Young Park S. (18)F-FDG PET/CT of advanced gastric carcinoma and association of HER2 expression with standardized uptake value. Asia Ocean J Nucl Med Biol 2014; 2:12-8
Celli R, Colunga M, Patel N, et al. Metabolic Signature on 18F-FDG PET/CT, HER2 Status, and Survival in Gastric Adenocarcinomas. J Nucl Med Technol 2016; 44:234-238
Acknowledgement
The study was supported by a grant from Beijing Municipal Natural Science Foundation [Nos. 7132037 and 7172043].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Research grants from funding agencies: Beijing Municipal Natural Science Foundation Grant funded by Beijing municipal commission of science and technology. Honoraria for speaking at symposia: no. Financial support for attending symposia: no. Financial support for educational programs: no. Employment or consultation: no. Support from a project sponsor: no. Position on advisory board or board of directors or other type of management relationships: no. Multiple affiliations: no. Financial relationships, for example, equity ownership or investment interest: no. Intellectual property rights (e.g., patents, copyrights, and royalties from such rights). Holdings of spouse and/or children that may have financial interest in the work: no.
Ethical standards
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Song, J., Li, Z., Chen, P. et al. The correlation between molecular pathological profiles and metabolic parameters of 18F-FDG PET/CT in patients with gastroesophageal junction cancer. Abdom Radiol 45, 312–321 (2020). https://doi.org/10.1007/s00261-019-02065-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-019-02065-5