Abstract
Purpose
To compare the predictive roles of qualitative (PI-RADSv2) and quantitative assessment (ADC metrics), in differentiating Gleason pattern (GP) 3 + 4 from the more aggressive GP 4 + 3 prostate cancer (PCa) using radical prostatectomy (RP) specimen as the reference standard.
Methods
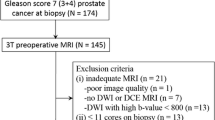
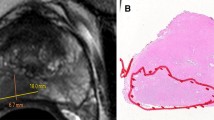
We retrospectively identified treatment-naïve peripheral (PZ) and transitional zone (TZ) Gleason Score 7 PCa patients who underwent multiparametric 3T prostate MRI (DWI with b value of 0,1400 and where unavailable, 0,500) and subsequent RP from 2011 to 2015. For each lesion identified on MRI, a PI-RADSv2 score was assigned by a radiologist blinded to pathology data. A PI-RADSv2 score ≤ 3 was defined as “low risk,” a PI-RADSv2 score ≥ 4 as “high risk” for clinically significant PCa. Mean tumor ADC (ADCT), ADC of adjacent normal tissue (ADCN), and ADCratio (ADCT/ADCN) were calculated. Stepwise regression analysis using tumor location, ADCT and ADCratio, b value, low vs. high PI-RADSv2 score was performed to differentiate GP 3 + 4 from 4 + 3.
Results
119 out of 645 cases initially identified met eligibility requirements. 76 lesions were GP 3 + 4, 43 were 4 + 3. ADCratio was significantly different between the two GP groups (p = 0.001). PI-RADSv2 score (“low” vs. “high”) was not significantly different between the two GP groups (p = 0.17). Regression analysis selected ADCT (p = 0.03) and ADCratio (p = 0.0007) as best predictors to differentiate GP 4 + 3 from 3 + 4. Estimated sensitivity, specificity, and accuracy of the predictive model in differentiating GP 4 + 3 from 3 + 4 were 37, 82, and 66%, respectively.
Conclusions
ADC metrics could differentiate GP 3 + 4 from 4 + 3 PCa with high specificity and moderate accuracy while PI-RADSv2, did not differentiate between these patterns.


Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A (2017) Cancer statistics, 2017. CA Cancer J Clin 67(1):7–30
Quinn DI, Henshall SM, Haynes AM, et al. (2001) Prognostic significance of pathologic features in localized prostate cancer treated with radical prostatectomy: implications for staging systems and predictive models. J Clin Oncol 19(16):3692–3705
Gleason DF, Mellinger GT (1974) Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol 111(1):58–64
Andrén O, Fall K, Franzén L, et al. (2006) How well does the Gleason score predict prostate cancer death? A 20-year followup of a population based cohort in Sweden. J Urol 175(4):1337–1340
Stark JR, Perner S, Stampfer MJ, et al. (2009) Gleason score and lethal prostate cancer: does 3 + 4 = 4 + 3? J Clin Oncol 27(21):3459–3464
Epstein JI, Zelefsky MJ, Sjoberg DD, et al. (2016) A contemporary prostate cancer grading system: a validated alternative to the Gleason score. Eur Urol 69(3):428–435
Kane CJ, Eggener SE, Shindel AW, Andriole GL (2017) Variability in outcomes for patients with intermediate-risk prostate cancer (Gleason Score 7, International Society of Urological Pathology Gleason Group 2–3) and implications for risk stratification: a systematic review. Eur Urol Focus 3(4–5):487–497
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA; Grading Committee (2016) The 2014 International Society of Urological Pathology (ISUP) consensus conference on gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol 40(2):244–252
Hoeks CMA, Barentsz JO, Hambrock T, et al. (2011) Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology 261(1):46–66
Jambor I, Boström PJ, Taimen P, et al. (2017) Novel biparametric MRI and targeted biopsy improves risk stratification in men with a clinical suspicion of prostate cancer (IMPROD Trial). J Magn Reson Imaging 46(4):1089–1095
Wu L-M, Xu J-R, Ye Y-Q, Lu Q, Hu J-N (2012) The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR 199(1):103–110
Weinreb JC, Barentsz JO, Choyke PL, et al. (2016) PI-RADS prostate imaging—reporting and data system: 2015, Version 2. Eur Urol 69(1):16–40
Hambrock T, Somford DM, Huisman HJ, et al. (2011) Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology 259(2):453–461
De Cobelli F, Ravelli S, Esposito A, et al. (2015) Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: comparison with prostate biopsy and radical prostatectomy specimen. AJR 204(3):550–557
Woo S, Kim SY, Cho JY, Kim SH (2016) Preoperative evaluation of prostate cancer aggressiveness: using ADC and ADC ratio in determining gleason score. AJR 207(1):114–120
Barrett T, Priest AN, Lawrence EM, et al. (2015) Ratio of tumor to normal prostate tissue apparent diffusion coefficient as a method for quantifying DWI of the prostate. AJR 205(6):W585–593
Litjens GJS, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ (2012) Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology 265(1):260–266
Peng Y, Jiang Y, Antic T, et al. (2014) Apparent diffusion coefficient for prostate cancer imaging: impact of b values. AJR 202(3):W247–253
Rosenkrantz AB, Triolo MJ, Melamed J, et al. (2014) Whole-lesion apparent diffusion coefficient metrics as a marker of percentage Gleason 4 component within Gleason 7 prostate cancer at radical prostatectomy. J Magn Reson Imaging 41(3):708–714
Itou Y, Nakanishi K, Narumi Y, Nishizawa Y, Tsukuma H (2011) Clinical utility of apparent diffusion coefficient (ADC) values in patients with prostate cancer: can ADC values contribute to assess the aggressiveness of prostate cancer? J Magn Reson Imaging 33(1):167–172
Barentsz JO, Richenberg J, Clements R, et al. (2012) ESUR prostate MR guidelines 2012. Eur Radiol 22(4):746–757
Park SY, Jung DC, Oh YT, et al. (2016) Prostate cancer: pI-RADS version 2 helps preoperatively predict clinically significant cancers. Radiology 280(1):151133
Hegde JV, Mulkern RV, Panych LP, et al. (2013) Multiparametric MRI of prostate cancer: an update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J Magn Reson Imaging 37(5):1035–1054
Hassanzadeh E, Alessandrino F, Olubiyi OI, et al. (2017) Comparison of quantitative apparent diffusion coefficient parameters with prostate imaging reporting and data system V2 assessment for detection of clinically significant peripheral zone prostate cancer. Abdom Radiol (NY) 43(5):1237–12425
Fennessy FM, Fedorov A, Penzkofer T, et al. (2015) Quantitative pharmacokinetic analysis of prostate cancer DCE-MRI at 3T: comparison of two arterial input functions on cancer detection with digitized whole mount histopathological validation. Magn Reson Imaging. 33(7):886–894
Srigley JR, Humphrey PA, Amin MB, et al. (2009) Protocol for the examination of specimens from patients with carcinoma of the prostate gland. Arch Pathol Lab Med 133(10):1568–1576
Buyyounouski MK, Choyke PL, Kattan MW, et al. (2017) Prostate. In: Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. (eds) AJCC Cancer Staging Manual, 8th edn. New York: Springer, pp 715–726
Nowak J, Malzahn U, Baur ADJ, et al. (2014) The value of ADC, T2 signal intensity, and a combination of both parameters to assess Gleason score and primary Gleason grades in patients with known prostate cancer. Acta Radiol 7(1):107–114
Jyoti R, Jain TP, Haxhimolla H, Liddell H, Barrett SE (2018) Correlation of apparent diffusion coefficient ratio on 3.0 T MRI with prostate cancer Gleason score. Eur J Radiol Open 5:58–63
Lebovici A, Sfrangeu SA, Feier D, et al. (2014) Evaluation of the normal-to-diseased apparent diffusion coefficient ratio as an indicator of prostate cancer aggressiveness. BMC Med Imaging 14:15
Itatani R, Namimoto T, Yoshimura A, et al. (2014) Clinical utility of the normalized apparent diffusion coefficient for preoperative evaluation of the aggressiveness of prostate cancer. Jpn J Radiol 32(12):685–691
Woo S, Suh CH, Kim SY, Cho JY, Kim SH (2018) Head-to-head comparison between high- and standard-b-value DWI for detecting prostate cancer: a systematic review and meta-analysis. AJR 210(1):91–100
Fennessy F, Fedorov A, Vangel M, et al. (2018) Multiparametric MRI as a biomarker of response to neoadjuvant second-generation hormone therapy for localized prostate cancer- a pilot study. Proc Intl Soc Mag Reson Med 2018:26
Hurrell SL, McGarry SD, Kaczmarowski A, et al. (2018) Optimized b-value selection for the discrimination of prostate cancer grades, including the cribriform pattern, using diffusion weighted imaging. J Med Imaging (Bellingham) 5(1):011004
Feng Z, Min X, Margolis DJA, et al. (2017) Evaluation of different mathematical models and different b-value ranges of diffusion-weighted imaging in peripheral zone prostate cancer detection using b-value up to 4500 s/mm2. Schwentner C, ed. PLoS ONE 12(2):e0172127
Purysko AS, Bittencourt LK, Bullen JA, et al. (2017) Accuracy and interobserver agreement for prostate imaging reporting and data system, version 2, for the characterization of lesions identified on multiparametric MRI of the prostate. AJR 209(2):339–349
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
National Institute of Health—National Cancer Institute: U01 CA151261; R25 CA089017; NIH P41EB 015898.
Conflict of interest
Francesco Alessandrino, Mehdi Taghipour, Elmira Hassanzadeh, Alireza Ziaei, and Mark Vangel declare they have no conflict of interest. Andriy Fedorov received the following grants from Institute of Health—National Cancer Institute: U01 CA151261; NIH P41EB 015898. Clare M Tempany received the following grants from Institute of Health—National Cancer Institute: R25 CA089017; NIH P41EB 015898. Fiona M Fennessy received the following grants from Institute of Health—National Cancer Institute: U01 CA151261; R25 CA089017; NIH P41EB 015898.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
For this type of study formal consent is not required.
Rights and permissions
About this article
Cite this article
Alessandrino, F., Taghipour, M., Hassanzadeh, E. et al. Predictive role of PI-RADSv2 and ADC parameters in differentiating Gleason pattern 3 + 4 and 4 + 3 prostate cancer. Abdom Radiol 44, 279–285 (2019). https://doi.org/10.1007/s00261-018-1718-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1718-6