Abstract
Purpose
Parametric response mapping (PRM) is a novel image-analysis technique applicable to assess tumor viability and predict intrahepatic recurrence of hepatocellular carcinoma (HCC) patients treated with transarterial chemoembolization (TACE). However, to date, the prognostic value of PRM for prediction of overall survival in HCC patients undergoing TACE is unclear. The objective of this explorative, single-center study was to identify cut-off values for voxel-specific PRM parameters that predict the post TACE overall survival in HCC patients.
Methods
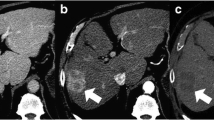
PRM was applied to biphasic CT data obtained at baseline and following 3 TACE treatments of 20 patients with HCC tumors ≥ 2 cm. The individual portal venous phases were registered to the arterial phases followed by segmentation of the largest lesion, i.e., the region of interest (ROI). Segmented voxels with their respective arterial and portal venous phase density values were displayed as a scatter plot. Voxel-specific PRM parameters were calculated and compared to patients’ survival at 1, 2, and 3 years post treatment to identify the maximal predictive parameters.
Results
The hypervascularized tissue portion of the ROI was found to represent an independent predictor of the post TACE overall survival. For this parameter, cut-off values of 3650, 2057, and 2057 voxels, respectively, were determined to be optimal to predict overall survival at 1, 2, and 3 years after TACE. Using these cut points, patients were correctly classified as having died with a sensitivity of 80, 92, and 86% and as still being alive with a specificity of 60, 75, and 83%, respectively. The prognostic accuracy measured by area under the curve (AUC) values ranged from 0.73 to 0.87.
Conclusion
PRM may have prognostic value to predict post TACE overall survival in HCC patients.







Similar content being viewed by others
Notes
Voxels with clear non-vital characteristics (density values > 300 HU arterial and > 100 HU portal venous) were filtered out [15].
Abbreviations
- AUC:
-
Area under the curve
- BCLC:
-
Barcelona Clinic Liver Cancer (staging system)
- cm:
-
Centimeter
- CT:
-
Computed tomography
- CI:
-
Confidence interval
- TACE:
-
Transarterial chemoembolization
- DEB:
-
Drug-eluting beads
- EC:
-
Ethics committee
- et al.:
-
And others
- i.e.,:
-
That means
- EASL:
-
European Association for the Study of Liver
- EORTC:
-
European Organisation for Research and Treatment of Cancer
- FOV:
-
Field of view
- HCC:
-
Hepatocellular carcinoma
- HU:
-
Hounsfield unit
- kVp:
-
Kilovoltage peak
- mAs:
-
Milliampere second
- MDCT:
-
Multi-detector computed tomography
- Mm:
-
Millimeter
- (m)RECIST:
-
(modified) Response Evaluation Criteria in Solid Tumors
- MRI:
-
Magnetic resonance imaging
- PRM:
-
Parametric Response Mapping
- ROC:
-
Receiver operating characteristic
- ROI:
-
Region of interest
- S:
-
Second
- SD:
-
Standard deviation
- SEN:
-
Sensitivity
- SIRT:
-
Selective internal radiotherapy
- SPE:
-
Specificity
- VIF:
-
Variance inflation factor
- WHO:
-
World Health Organization
References
Kudo M, Trevisani F, Abou-Alfa GK, Rimassa L (2016) Hepatocellular carcinoma: therapeutic guidelines and medical treatment. Liver Cancer 6:16–26. https://doi.org/10.1159/000449343
Huang Z, Zhang N, Li W, et al. (2017) Expression of CHODL in hepatocellular carcinoma affects invasion and migration of liver cancer cells. Oncol Lett 13:715–721. https://doi.org/10.3892/ol.2016.5466
Ferlay J, Shin H-R, Bray F, et al. (2010) Estimates of worldwide burden of cancer in 2008: gLOBOCAN 2008. Int J Cancer 127:2893–2917. https://doi.org/10.1002/ijc.25516
World Health Organization (2012) Liver Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 11 Oct 2016
European Association for the Study of the Liver, European Organisation for Research and Treatment of Cancer (2012) EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–943. https://doi.org/10.1016/j.jhep.2011.12.001
National Comprehensive Cancer Network (2015) NCCN clinical practice guidelines in oncology (NCCN Guidelines®): hepatobiliary cancers. J Natl Compr Cancer Netw 7(4): 350–391
Llovet JM, Bruix J (2003) Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology 37:429–442. https://doi.org/10.1053/jhep.2003.50047
Llovet JM, Real MI, Montaña X, et al. (2002) Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet 359:1734–1739. https://doi.org/10.1016/S0140-6736(02)08649-X
Choi SJ, Kim J, Kim HS, Park H (2017) Parametric response mapping of dynamic CT: enhanced prediction of survival in hepatocellular carcinoma patients treated with transarterial chemoembolization. Abdom Radiol N Y 42:1871–1879. https://doi.org/10.1007/s00261-017-1082-y
Shim JH, Lee HC, Kim S-O, et al. (2012) Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? a validation study of old and new models. Radiology 262:708–718. https://doi.org/10.1148/radiol.11110282
Lencioni R, Llovet JM (2010) Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60. https://doi.org/10.1055/s-0030-1247132
Gillmore R, Stuart S, Kirkwood A, et al. (2011) EASL and mRECIST responses are independent prognostic factors for survival in hepatocellular cancer patients treated with transarterial embolization. J Hepatol 55:1309–1316. https://doi.org/10.1016/j.jhep.2011.03.007
Vincenzi B, Di Maio M, Silletta M, et al. (2015) Prognostic relevance of objective response according to EASL criteria and mRECIST criteria in hepatocellular carcinoma patients treated with loco-regional therapies: a literature-based meta-analysis. PLoS One 10:e0133488. https://doi.org/10.1371/journal.pone.0133488
Bonekamp S, Halappa VG, Geschwind J-FH, et al. (2013) Unresectable hepatocellular carcinoma: MR imaging after intraarterial therapy. Part ii. response stratification using volumetric functional criteria after intraarterial therapy. Radiology 268:431–439. https://doi.org/10.1148/radiol.13121637
Hinrichs JB, Shin H-O, Kaercher D, et al. (2016) Parametric response mapping of contrast-enhanced biphasic CT for evaluating tumour viability of hepatocellular carcinoma after TACE. Eur Radiol 26:3447–3455. https://doi.org/10.1007/s00330-015-4203-4
Choi SJ, Kim J, Seo J, et al. (2014) Parametric response mapping of dynamic CT as an imaging biomarker to distinguish viability of hepatocellular carcinoma treated with transcatheter arterial chemoembolization. Abdom Imaging 39:518–525. https://doi.org/10.1007/s00261-014-0087-z
Galbán CJ, Chenevert TL, Meyer CR, et al. (2009) The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med 15:572–576. https://doi.org/10.1038/nm.1919
Choi SJ, Kim J, Seo J, et al. (2016) Parametric response mapping of dynamic CT for predicting intrahepatic recurrence of hepatocellular carcinoma after conventional transcatheter arterial chemoembolization. Eur Radiol 26:225–234. https://doi.org/10.1007/s00330-015-3825-x
Granata V, Fusco R, Catalano O, et al. (2017) Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE 12:e0179951. https://doi.org/10.1371/journal.pone.0179951
Avants B, Epstein C, Grossman M, Gee J (2008) Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal 12:26–41. https://doi.org/10.1016/j.media.2007.06.004
Andersen PK, Gill RD (1982) Cox’s Regression Model for Counting Processes: a Large Sample Study. Ann Stat 10:1100–1120. https://doi.org/10.1214/aos/1176345976
Therneau TM, Grambsch PM (2000) Modeling Survival Data: Extending the Cox Model, 1st edn. New York: Springer
Therneau TM (2015) A Package for Survival Analysis in S. version 2.38
Kutner MH, Nachtsheim CJ, Neter J (2004) Applied Linear Regression Models, 4th edn. New York: McGraw-Hill Education
Core Team R (2016) R: A Language and Environment for Statistical Computing. Vienna: Austria
Sing T, Sander O, Beerenwinkel N, Lengauer T (2005) ROCR: visualizing classifier performance in R. Bioinformatics 21:3940–3941. https://doi.org/10.1093/bioinformatics/bti623
Riddle DL, Stratford PW (1999) Interpreting Validity Indexes for Diagnostic Tests: an Illustration Using the Berg Balance Test. Phys Ther 79:939–948
Gallop RJ, Crits-Christoph P, Muenz LR, Tu XM (2003) Determination and Interpretation of the Optimal Operating Point for ROC Curves Derived Through Generalized Linear Models. Understanding Statistics 2:219–242. https://doi.org/10.1207/S15328031US0204_01
Lopez-Raton M, Rodriguez-Alvarez MX, Suarez CC, Sampedro FG (2014) OptimalCutpoints: an R Package for Selecting Optimal Cutpoints in Diagnostic Tests. Journal of Statistical Software 61:1–36
Arora A, Kumar A (2014) Treatment Response Evaluation and Follow-up in Hepatocellular Carcinoma. J Clin Exp Hepatol 4:126–129. https://doi.org/10.1016/j.jceh.2014.05.005
Subbiah V, Chuang HH, Gambhire D, Kairemo K (2017) Defining Clinical Response Criteria and Early Response Criteria for Precision Oncology: current State-of-the-Art and Future Perspectives. Diagnostics (Basel) . https://doi.org/10.3390/diagnostics7010010
Bargellini I, Bozzi E, Campani D, et al. (2013) Modified RECIST to assess tumor response after transarterial chemoembolization of hepatocellular carcinoma: cT-pathologic correlation in 178 liver explants. Eur J Radiol 82:e212–218. https://doi.org/10.1016/j.ejrad.2012.12.009
El-Gazzaz G, Sourianarayanane A, Menon KVN, et al. (2013) Radiologic-histological correlation of hepatocellular carcinoma treated via pre-liver transplant locoregional therapies. Hepatobiliary Pancreat Dis Int 12:34–41
Forner A, Ayuso C, Varela M, et al. (2009) Evaluation of tumor response after locoregional therapies in hepatocellular carcinoma: are response evaluation criteria in solid tumors reliable? Cancer 115:616–623. https://doi.org/10.1002/cncr.24050
Llovet JM, Ricci S, Mazzaferro V, et al. (2008) Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 359:378–390. https://doi.org/10.1056/NEJMoa0708857
Liu L, Wang W, Chen H, et al. (2014) EASL- and mRECIST-evaluated responses to combination therapy of sorafenib with transarterial chemoembolization predict survival in patients with hepatocellular carcinoma. Clin Cancer Res 20:1623–1631. https://doi.org/10.1158/1078-0432.CCR-13-1716
Willatt JM, Hussain HK, Adusumilli S, Marrero JA (2008) MR Imaging of Hepatocellular Carcinoma in the Cirrhotic Liver: challenges and Controversies. Radiology 247:311–330. https://doi.org/10.1148/radiol.2472061331
Kim SH, Lee WJ, Lim HK, Lim JH (2007) Prediction of viable tumor in hepatocellular carcinoma treated with transcatheter arterial chemoembolization: usefulness of attenuation value measurement at quadruple-phase helical computed tomography. J Comput Assist Tomogr 31:198–203. https://doi.org/10.1097/01.rct.0000236424.20514.2e
Hunt SJ, Yu W, Weintraub J, et al. (2009) Radiologic monitoring of hepatocellular carcinoma tumor viability after transhepatic arterial chemoembolization: estimating the accuracy of contrast-enhanced cross-sectional imaging with histopathologic correlation. J Vasc Interv Radiol 20:30–38. https://doi.org/10.1016/j.jvir.2008.09.034
Lamba R, McGahan JP, Corwin MT, et al. (2014) CT Hounsfield Numbers of Soft Tissues on Unenhanced Abdominal CT Scans: variability Between Two Different Manufacturers’ MDCT Scanners. AJR Am J Roentgenol 203:1013–1020. https://doi.org/10.2214/AJR.12.10037
Acknowledgements
We would like to thank Philip D. Walson, MD, Visiting Professor, Department of Clinical Pharmacology, University Medical Center Göttingen, Germany for providing English language editing of draft versions of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Animal ethical approval
This article does not contain any studies with animals performed by any of the authors.
Human ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. For this type of study formal consent was not required.
Informed consent
Informed consent was obtained from all individual participants included in the study for the medical and radiologic procedures performed.
Rights and permissions
About this article
Cite this article
Nörthen, A., Asendorf, T., Shin, HO. et al. Parametric response mapping cut-off values that predict survival of hepatocellular carcinoma patients after TACE. Abdom Radiol 43, 3288–3300 (2018). https://doi.org/10.1007/s00261-018-1610-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00261-018-1610-4