Abstract
Purpose
Prostate-Specific Membrane Antigen (PSMA)-targeted Positron Emission Tomography (PET) has revolutionised prostate cancer (PCa) diagnosis and treatment, offering superior diagnostic accuracy over traditional methods and enabling theragnostic applications. However, a significant diagnostic challenge has emerged with identifying unspecific bone uptakes (UBUs), which could lead to over-staging and inappropriate treatment decisions if misinterpreted. This systematic review explores the phenomenon of UBUs in PCa patients undergoing PSMA-PET imaging.
Methods
Studies assessing the prevalence, topographical distribution, and potential clinical implications of UBUs were selected according to the Preferred Reporting Items for a Systematic Review and Meta-Analysis (PRISMA) method and evaluated with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.
Results
The percentage of PCa patients with UBUs on PSMA-PET scans ranged from 0 to 71.7%, depending on the radiopharmaceutical used, with [18F]PSMA-1007 showing the highest incidence. The ribs are the primary site of UBUs across all PSMA-targeted radiopharmaceuticals. The spine is the second most frequent UBU site for [68Ga]Ga-PSMA-11, [18F]DCFPyL, [18F]rhPSMA-7, while the pelvic girdle represents the second most frequent site for [18F]PSMA-1007. The average maximum Standardized Uptake Value (SUVmax) of UBUs varied from 3.4 to 7.7 and was generally lower than that of bone metastases.
Conclusions
Our findings underscore the need for heightened awareness and precise interpretation of UBUs to avoid potential over-staging and subsequent inappropriate treatment decisions. Considering the radiopharmaceutical used, PET-derived semiquantitative parameters, the topographical distribution of UBUs, and accurately evaluating the pre-test probability based on clinical and laboratory parameters may aid nuclear medicine physicians in interpreting PSMA-PET findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recent advances in prostate cancer (PCa) management have been significantly influenced by the advantages of PSMA-targeted PET scans over traditional diagnostics, paving the way for their use as theragnostic agents [1]. Despite initial treatments like radiation or surgery, up to 60% of PCa patients can face biochemical recurrence (BCR) within a decade. Early identification of disease sites enables targeted interventions such as local salvage therapy for relapses or metastatic ablation for oligometastatic PCa, providing possible curative alternatives to palliative androgen-deprivation therapy [2, 3].
Considering the high expression of PSMA on the cell membrane of PCa cells and based on the first urea-based compounds, several low-molecular-weight radiolabelled PSMA inhibitors have been developed to expand the diagnostic performance of nuclear medicine imaging for PCa detection. Currently, the most commonly used PSMA-targeting radiopharmaceutical worldwide is [68Ga]Ga-PSMA-11, also known as [68Ga]Ga-DKFZ-PSMA-11 or [68Ga]Ga-PSMA-HBED-CC [4,5,6,7]. Recently, [18F]labeled PSMA-targeting radiopharmaceuticals were widely adopted into clinical practice, mainly with [18F]DCFPyL and [18F]PSMA-1007 [8, 9]. Unlike other PSMA radioligands, [18F]PSMA-1007 has increased lipophilicity and is primarily eliminated by the liver. This characteristic may reduce nonspecific activity in the ureter and bladder, potentially mitigating urinary excretion issues [10]. [18F]-labelled options potentially offer reduced costs, broadened availability, and superior image quality due to lower positron energy [11]. However, with the progressive increase in the number of facilities performing PSMA-targeted PET worldwide and the expanding body of literature, a limitation of this relatively novel diagnostic probe is represented by unspecific bone uptakes (UBUs) [12]. If misinterpreted, these false positive findings could result in PCa over-staging and lead to erroneous treatment choices (i.e., palliative over radical therapy).
Several studies have recently investigated the incidence of UBUs in PET imaging using different PSMA-targeting radiopharmaceuticals among PCa patients across various clinical settings. This systematic review aims to collect the available literature on this PSMA-PET potential drawback, highlight the main differences among the most used radiopharmaceuticals, and summarise the topographical quantitative distribution of these findings.
Materials and methods
Protocol, review question and inclusion criteria
Based on a preconceived protocol [13], the current systematic review was developed referring to the “Preferred Reporting Items for a Systematic Review and Meta-Analysis” (PRISMA 2020 statement) [14]. The comprehensive PRISMA checklist is available in the Supplementary Table 1. The systematic review has been preregistered on the PROSPERO database (protocol number CRD42024519876).
A review question was defined based on the “Population, Intervention, Comparator, Outcomes” framework (PICO): what is the prevalence of UBUs (outcome) on PSMA-targeted PET imaging (intervention) in patients diagnosed with PCa (patient/population)? The presence of a comparator was not considered an exclusion criterion. Two authors (M.B. and A.R.) independently conducted the literature search, study selection, quality assessment, and data extraction. Disagreements were resolved through an online meeting with a third reviewer (S.M.). Reviews, editorials, comments, case reports, and original investigations on different topics were excluded. No language restriction was applied.
Literature search strategy, selection process, data collection and extraction
The authors comprehensively searched for articles dealing with UBUs on PSMA-targeted PET images, employing two electronic bibliographic databases (Scopus and PubMed/MEDLINE). The search algorithm included the following terms: (“PSMA” OR “Prostate Specific Membrane Antigen”) AND (“unspecific” OR “not specific” OR “non-specific” OR “nonspecific” OR “indeterminate” OR “undetermined” OR “uncertain” OR “unclear” OR “UBU”) AND (“bone” OR “skelet*”)). Moreover, reviewers screened included studies’ references, searching for additional eligible articles meeting the predetermined inclusion criteria. The literature search was last updated on 25.02.2024.
The reviewers independently read the titles and abstracts of the records generated by the search algorithm. They then determined which studies were eligible based on predefined criteria. Thereafter, the reviewers collected data from all of the included studies, taking advantage of full-text, tables, and supplemental material regarding general study information (authors, publication year, country, study design, funding sources), patients’ characteristics (sample size, age, clinical setting, Gleason score, serum markers levels), PET-related details (administered radiopharmaceuticals and their activity, hybrid imaging protocol, image analysis method), and outcome (including UBU prevalence, UBU sites, average radiopharmaceutical uptake for UBU, and UBU validation method).
Quality assessment (risk of bias assessment)
QUADAS-2 was used to assess the quality of the included studies, to analyse the risk of bias, and to determine their pertinence to the review question [15]. To perform the quality assessment, the reviewers considered four domains (patient selection, index test, reference standard, flow, and timing). To assess the applicability of the included studies, they considered three categories (patient selection, index test, and reference standard).
Literature analysis
Due to the heterogeneity of the available studies and the absence of quantitative data in more cases, we planned a systematic review (qualitative synthesis) without a meta-analysis (quantitative synthesis). Therefore, a statistical analysis (pooled analysis) was not performed.
Results
Study characteristics
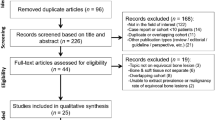
Fifteen studies satisfied the inclusion criteria [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The study selection process is summarized in Fig. 1. All the included articles except two accounted for a retrospective design [16,17,18,19,20,21,22,23, 25, 27,28,29,30], whereas the remaining trials were prospective [24, 26]. Only two papers reported a multicentric design [22, 27]. Table 1 summarises the general information of the included studies.
Table 2 presents the clinical characteristics of PCa patients from various studies. The number of participants varied from 10 to 792 (age range: 67-72.1). In four studies, PSMA PET was used for restaging PCa patients [18, 19, 27, 29], while two studies were conducted for primary staging [17, 28]. The remaining nine studies utilised PSMA PET imaging in both contexts [16, 20,21,22,23,24,25,26, 30]. When provided, the Gleason score for grading the included patients was reported as International Society of Urological Pathology (ISUP) Grade Group 1 in two patients, ISUP 2 in 576 patients, and ISUP 3 in 399 patients [16, 18, 20, 21, 23,24,25,26,27,28]. Regarding prostate-specific antigen (PSA) serum levels, the average values reported ranged from 0.8 to 110 ng/mL [19].
Technical details of the included studies are reported in Table 3. All included studies qualitatively evaluated UBUs, with thirteen conducting a semiquantitative analysis to extract the standardised uptake values (SUV) [16, 18, 20,21,22,23,24,25,26,27,28,29,30].
Seven studies conducted bone biopsies on at least one patient regarding the reference standard used to assess the aetiology of focal bone uptakes [17, 20, 22, 25,26,27, 30], while ten studies used a composite reference standard with or without biopsy [16,17,18,19,20, 22, 26, 27, 29, 30]. Four studies lacked methods to verify if bone uptakes were UBUs or misdiagnosed metastases [21, 23, 24, 28].
Risk of bias and applicability
Reviewers used the QUADAS-2 tool to assess the relevance of each paper based on reported data. Figure 2 briefly resumes the concerns about the quality and applicability of the included research.

Authors classified the papers included in the systematic review as high- or low-risk of bias or applicability concerns for distinct domains listed in the ordinate axis. The graph indicates that almost 40% of the included studies are affected by a high risk of bias in the “reference standard” domain
Quality assessment according to QUADAS-2 tool.
Results of individual studies (qualitative synthesis)
When assessed, the percentage of PCa patients with UBUs ranged from 11.6 to 71.7% for [18F]PSMA-1007 [18,19,20,21,22, 24, 28,29,30], from 0 to 23.9% for [68Ga]Ga-PSMA-11 [16, 18, 21, 24, 29], and was 19.8% for the single study using [18F]DCFPyl [26]. Concerning the semiquantitative metrics, the average UBUs SUVmax values varied from 3.4 to 7.7 for [18F]PSMA-1007 and 4.6 to 5 for [68Ga]Ga-PSMA-11. When reported, UBUs uptake was significantly lower than bone metastases [20, 24, 26, 27, 30]. UBUs incidence and uptake characteristics reported by the selected studies are summarized in Table 4.
One study assessed the differences in the incidence of UBUs by comparing PET scans performed in different centres and observed a significantly higher number of UBUs in digital than analogue PET [22]. The same paper correlated the uptake time with the incidence of UBUs [22].
Regarding the anatomical localisation, most UBUs were reported in the ribs, spine and pelvic girdle, followed by the sternum, shoulder girdle and limbs [16,17,18, 20,21,22,23,24,25,26,27,28,29,30]. A less frequent UBUs location was the skull. In Fig. 3, we illustrate the topographical distribution of [18F]PSMA-1007 UBUs throughout the entire skeleton, drawing inspiration from Penfield’s human homunculus [31]. However, minor differences in UBU topography can be observed when comparing different PSMA-targeted tracers. A quantitative synthesis of the prominent UBU locations according to the PSMA-ligand used across the included studies is reported in Table 5 and visually represented in Fig. 4.

This figure provides a visual representation of the distribution of UBUs throughout the human skeleton according to the PSMA-targeted radiopharmaceutical used, emphasizing high-incidence areas with hot colours to underscore their clinical significance
The distribution of UBUs according to the PSMA-targeted radiopharmaceutical used.
When biopsied, UBUs were mainly related to benign conditions such as fibroblastic reaction, fibrous dysplasia, hyperplastic bone marrow, or Paget’s disease. Interestingly, some papers did not report any alteration in the biopsied bone marrow [17, 20, 22, 25,26,27, 30] (Table 4).
Several studies aimed to identify clinical risk factors associated with the occurrence of UBUs in PET scans. No relationship was observed between the frequency of UBUs and the clinical indication for the PET scan (primary staging vs. restaging). Additionally, serum PSA levels and Gleason Score were not considered risk factors for the appearance of UBUs [16, 20, 22, 27]. Ninatti et al. explored a potential correlation between the presence of UBUs and elevated white blood cell counts [28]. The same study also observed lower body mass index and bone density values (measured in Hounsfield Units), in patients presenting UBUs, though these findings were not statistically significant [28]. Given that focal uptakes in bones might not only represent false-positive findings but could also reflect the presence of bone metastases, numerous studies focused on the role of clinical, biochemical, and histopathological features in differentiating between benign and malignant causes of these uptakes. Within this context, PSA levels and PCa histology have emerged as potentially valuable indicators [20, 24, 26, 27, 30].
Discussion
The present systematic review highlights the complexity of the UBUs phenomenon in PET/CT scans utilising PSMA-targeted ligands. Consistent with prior literature [29, 32], we identified [18F]PSMA-1007 as the tracer associated with a significantly higher rate of UBUs, especially in rib areas, across all examined settings. This topic is of increasing relevance, as the clinical use of [18F]PSMA-1007 is rising due to several reasons, including cyclotron-based production (which allows synthesising larger quantities of [18F]PSMA compared to [68Ga]Ga generators), the longer half-life, the lower positron range and the higher signal-to-background ratio [18, 33]. Despite [18F]PSMA-1007 being the tracer most frequently related to the presence of UBUs, renally excreted radiopharmaceuticals might also be associated with this phenomenon. Indeed, in the included studies, the percentage of patients with equivocal bony findings ranged from 0 to 23.9% [16, 18, 21, 24, 26, 29]. Similarly, in the OSPREY trial, which included a bone biopsy for 44 patients undergoing restaging for disease recurrence with [18F]DCFPyL, false positive findings were observed in about 15% of the patients included [34]. .
The precise pathophysiological mechanisms behind UBUs remain elusive. The initial hypothesis of free fluorine involvement has been challenged [35], with the chemical composition-driven affinity being partially responsible [36]. Nevertheless, PSMA radioligands with hydrophilic compositions also demonstrate false positive bone findings, suggesting this phenomenon is not unique to [18F]PSMA-1007 [29]. Furthermore, healthy bone marrow lacks PSMA immunohistochemical positivity, highlighting the limited understanding of these unspecific uptakes’ biological mechanisms [37]. However, at least in some instances, a morphological correlate seems likely, given that UBUs may persist in follow-up scans [22]; this calls for further research to elucidate their nature. As observed by the groups of Alberts [12] and Grünig [22], the technological shift towards digital PET devices introduces a further bias towards increased false positive findings. Notably, when comparing digital PET/MRI with analogue PET/CT scanners, the difference in false positive findings was not observed, perhaps due to the slightly reduced sensitivity of digital PET detectors in MRI scanners due to coils and the magnetic field [38]. The evolving technological landscape thus necessitates awareness of the trade-offs between sensitivity and specificity. Texture analysis of lesions emerges as a potential game-changer for differential diagnosis, although its practical application requires substantial datasets [39].
From a clinical perspective, the misinterpretation of UBUs can lead to inappropriate treatment decisions, intensifying the ongoing discussion about the stage migration phenomenon in cancer diagnosis and treatment [39,40,41]. In this regard, our systematic review focused on understanding the topography of UBUs by differentiating between PSMA-targeted ligands, thereby enhancing our knowledge base for image interpretation. Our primary finding is that the ribs are the most frequent site of UBUs, irrespective of the PSMA-targeted radiopharmaceutical used. Wang et al. [42] previously explored the distribution of bone metastases in a large cohort of PCa patients through bone scans, noting a predominant occurrence in the vertebrae and pelvis during the early stages [42]. Their study revealed that only 1% of patients exhibited bone metastases without involvement of the vertebrae and pelvis [42]. Given the differences in topography between UBUs and typical PCa bone metastatic patterns, we suggest that a single PSMA-avid focal uptake in the ribs is unlikely to be metastatic in most cases, regardless of the PSMA radiopharmaceutical used. In contrast, focal bone uptake involving the spine (the second most frequent UBU site for [68Ga]Ga-PSMA-11, [18F]DCFPyL, [18F]rhPSMA-7) or the pelvic girdle (the second most frequent site for [18F]PSMA-1007) presents more interpretive challenges. In these cases, analyzing imaging parameters may improve image interpretation. Indeed, some studies have focused on SUVmax [20, 24, 26, 27, 30], even with the goal of establishing a cutoff value for clinical decision-making [20]. Further research identifying clinical risk factors for bone metastases in patients with focal bone uptakes underscores the importance of integrating clinical context with imaging. This context includes factors such as PSA levels, histology [20, 24, 26, 27, 30] and even non-cancer-related parameters like white blood cell counts [28]. These insights suggest that nuclear medicine physicians must sometimes tailor their reports based on the radiopharmaceutical used, prioritizing pre-test metastatic probability over individual uptakes.
This systematic review acknowledges several limitations: (i) the heterogeneity of the included studies and the nature of the topic precluded data pooling and a meta-analysis. Merging prevalence rates from studies with varying inclusion criteria and different radiopharmaceuticals could lead to misleading outcomes; (ii) variability in reported data prevented a per-lesion analysis; (iii) our tracer-specific topographical assessment of UBUs is limited to data from only four PSMA-targeted tracers, due to the scarcity of comprehensive data on this topic in the current literature; (iv) most of the included studies were retrospective, and most patients did not undergo confirmatory biopsy of their UBUs, making it difficult to accurately determine the proportion of false positives versus true bone metastases. This limitation introduces a potential bias that should not be overlooked. Furthermore, although this review did not directly assess the preferential use of one radiopharmaceutical over another in clinical practice, it included a prospective study comparing [18F]PSMA-1007 and [68Ga]Ga-PSMA-11 [24], which suggests the equivalent performance of these tracers in nodal and distant metastasis staging. Additionally, a retrospective study by Seifert et al. [29] involving BCR patients indicated no significant difference in the detection rates of bone metastases between the two radiopharmaceuticals. This supports the premise that experienced physicians can effectively adjust for UBU findings, emphasizing the flexibility in the choice of PSMA-targeted agents in clinical practice. Finally, this review focused on radiopharmaceuticals labelled with positron emitters and excluded gamma-emitting tracers, such as [99mTc]Tc-labeled PSMA radiopharmaceuticals, and PSMA-targeted radioligands used for therapeutic purposes, such as [177Lu]Lu-PSMA-617. The exclusion is due to the absence of reports in the available literature concerning UBUs observed in both diagnostic and post-treatment scintigraphic imaging.
Conclusions
In conclusion, UBUs present a notable diagnostic challenge in a diverse range of patients undergoing PSMA PET scans for PCa, especially when using [18F]PSMA-1007. From our systematic review, we draw the following key conclusions:
-
The ribs are the primary site of UBUs across all PSMA-targeted radiopharmaceuticals. Isolated rib uptakes are typically non-metastatic.
-
Focal bone uptakes involving the spine or pelvic girdle, the second most frequent UBU sites for specific PSMA tracers, require careful interpretation due to their complexity.
-
Evaluating imaging parameters, such as SUVmax, in conjunction with clinical context assessment is crucial for accurately interpreting challenging cases.
Data availability
N.A.
References
Bauckneht M, Ciccarese C, Laudicella R, Mosillo C, D’Amico F, Anghelone A, et al. Theranostics revolution in prostate cancer: basics, clinical applications, open issues and future perspectives. Cancer Treat Rev. 2024;124:102698.
Mullins JK, Feng Z, Trock BJ, Epstein JI, Walsh PC, Loeb S. The impact of anatomical radical retropubic prostatectomy on cancer control: the 30-year anniversary. J Urol. 2012;188:2219–24.
Phillips R, Shi WY, Deek M, Radwan N, Lim SJ, Antonarakis ES, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for oligometastatic prostate Cancer: the ORIOLE phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6:650–9.
Eder M, Schäfer M, Bauder-Wüst U, Hull W-E, Wängler C, Mier W, et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688–97.
Afshar-Oromieh A, Haberkorn U, Eder M, Eisenhut M, Zechmann CM. [68Ga]Gallium-labelled PSMA ligand as superior PET tracer for the diagnosis of prostate cancer: comparison with 18F-FECH. Eur J Nucl Med Mol Imaging. 2012;39:1085–6.
Afshar-Oromieh A, Malcher A, Eder M, Eisenhut M, Linhart HG, Hadaschik BA, et al. PET imaging with a [68Ga]gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–95.
Wester H-J, Schottelius M. PSMA-Targeted Radiopharmaceuticals for Imaging and Therapy. Semin Nucl Med. 2019;49:302–12.
Cardinale J, Schäfer M, Benešová M, Bauder-Wüst U, Leotta K, Eder M, et al. Preclinical evaluation of 18F-PSMA-1007, a New Prostate-Specific Membrane Antigen Ligand for prostate Cancer imaging. J Nucl Med. 2017;58:425–31.
Giesel FL, Will L, Lawal I, Lengana T, Kratochwil C, Vorster M, et al. Intraindividual comparison of 18F-PSMA-1007 and 18F-DCFPyL PET/CT in the prospective evaluation of patients with newly diagnosed prostate carcinoma: a pilot study. J Nucl Med. 2018;59:1076–80.
Bauckneht M, Miceli A, Signori A, Albano D, Capitanio S, Piva R, et al. Combined forced diuresis and late acquisition on [68Ga]Ga-PSMA-11 PET/CT for biochemical recurrent prostate cancer: a clinical practice-oriented study. Eur Radiol. 2023;33:3343–53.
Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, et al. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44:678–88.
Alberts IL, Seide SE, Mingels C, Bohn KP, Shi K, Zacho HD, et al. Comparing the diagnostic performance of radiotracers in recurrent prostate cancer: a systematic review and network meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:2978–89.
Sadeghi R, Treglia G. Systematic reviews and meta-analyses of diagnostic studies: a practical guideline. Clin Transl Imaging. 2017;5:83–7.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised Tool for the Quality Assessment of Diagnostic Accuracy studies. Ann Intern Med. 2011;155:529–36.
Chiu LW, Lawhn-Heath C, Behr SC, Juarez R, Perez PM, Lobach I, et al. Factors Predicting Metastatic Disease in 68Ga-PSMA-11 PET-Positive osseous lesions in prostate Cancer. J Nucl Med. 2020;61:1779–85.
Chen MY, Franklin A, Yaxley J, Gianduzzo T, McBean R, Wong D, et al. Solitary rib lesions showing prostate-specific membrane antigen (PSMA) uptake in pre-treatment staging 68 Ga-PSMA-11 positron emission tomography scans for men with prostate cancer: benign or malignant? BJU Int. 2020;126:396–401.
Rauscher I, Krönke M, König M, Gafita A, Maurer T, Horn T, et al. Matched-pair comparison of 68Ga-PSMA-11 PET/CT and 18F-PSMA-1007 PET/CT: frequency of pitfalls and detection efficacy in biochemical recurrence after radical prostatectomy. J Nucl Med. 2020;61:51–7.
Dietlein F, Kobe C, Hohberg M, Zlatopolskiy BD, Krapf P, Endepols H, et al. Intraindividual comparison of 18F-PSMA-1007 with Renally Excreted PSMA ligands for PSMA PET Imaging in patients with relapsed prostate Cancer. J Nucl Med. 2020;61:729–34.
Arnfield EG, Thomas PA, Roberts MJ, Pelecanos AM, Ramsay SC, Lin CY, et al. Clinical insignificance of [18F]PSMA-1007 avid non-specific bone lesions: a retrospective evaluation. Eur J Nucl Med Mol Imaging. 2021;48:4495–507.
Hoberück S, Löck S, Borkowetz A, Sommer U, Winzer R, Zöphel K, et al. Intraindividual comparison of [68 Ga]-Ga-PSMA-11 and [18F]-F-PSMA-1007 in prostate cancer patients: a retrospective single-center analysis. EJNMMI Res. 2021;11:109.
Grünig H, Maurer A, Thali Y, Kovacs Z, Strobel K, Burger IA, et al. Focal unspecific bone uptake on [18F]-PSMA-1007 PET: a multicenter retrospective evaluation of the distribution, frequency, and quantitative parameters of a potential pitfall in prostate cancer imaging. Eur J Nucl Med Mol Imaging. 2021;48:4483–94.
Kroenke M, Mirzoyan L, Horn T, Peeken JC, Wurzer A, Wester H-J, et al. Matched-pair comparison of 68Ga-PSMA-11 and 18F-rhPSMA-7 PET/CT in patients with primary and biochemical recurrence of prostate Cancer: frequency of non-tumor-related uptake and Tumor Positivity. J Nucl Med. 2021;62:1082–8.
Pattison DA, Debowski M, Gulhane B, Arnfield EG, Pelecanos AM, Garcia PL, et al. Prospective intra-individual blinded comparison of [18F]PSMA-1007 and [68 Ga]Ga-PSMA-11 PET/CT imaging in patients with confirmed prostate cancer. Eur J Nucl Med Mol Imaging. 2022;49:763–76.
Vollnberg B, Alberts I, Genitsch V, Rominger A, Afshar-Oromieh A. Assessment of malignancy and PSMA expression of uncertain bone foci in [18F]PSMA-1007 PET/CT for prostate cancer-a single-centre experience of PET-guided biopsies. Eur J Nucl Med Mol Imaging. 2022;49:3910–6.
Phelps TE, Harmon SA, Mena E, Lindenberg L, Shih JH, Citrin DE, et al. Predicting outcomes of Indeterminate Bone lesions on 18F-DCFPyL PSMA PET/CT scans in the setting of high-risk primary or recurrent prostate Cancer. J Nucl Med. 2023;64:395–401.
Letang A, Crombé A, Rousseau C, Sargos P, Merlin C, Cantarel C, et al. Bone uptake in prostate Cancer patients: Diagnostic performances of PSMA-RADS v1.0, Clinical, Biological, and 68 Ga-PSMA-11 PET features to Predict Metastasis after biochemical recurrence. Clin Nucl Med. 2022;47:e529–39.
Ninatti G, Pini C, Gelardi F, Ghezzo S, Mapelli P, Picchio M, et al. The potential role of osteoporosis in unspecific [18F]PSMA-1007 bone uptake. Eur J Nucl Med Mol Imaging. 2023;51:304–11.
Seifert R, Telli T, Opitz M, Barbato F, Berliner C, Nader M, et al. Unspecific 18F-PSMA-1007 bone uptake evaluated through PSMA-11 PET, bone scanning, and MRI triple validation in patients with biochemical recurrence of prostate Cancer. J Nucl Med. 2023;64:738–43.
Luo L, Wang Z, Wang X, Gao J, Zheng A, Duan X. Fluorine-18 prostate-specific membrane antigen-1007-avid indeterminate bone lesions in prostate cancer: clinical and PET/CT features to predict outcomes and prognosis. Clin Radiol. 2024;S0009–9260(24):00001–1.
Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443.
Woo S, Freedman D, Becker AS, Leithner D, Mayerhoefer ME, Friedman KP, et al. Equivocal bone lesions on PSMA PET/CT: systematic review and meta-analysis on their prevalence and malignancy rate. Clin Transl Imaging. 2024. https://doi.org/10.1007/s40336-024-00631-6.
Evangelista L, Maurer T, van der Poel H, Alongi F, Kunikowska J, Laudicella R, et al. [68Ga]Ga-PSMA Versus [18F]PSMA Positron Emission Tomography/Computed Tomography in the staging of primary and recurrent prostate Cancer. A systematic review of the literature. Eur Urol Oncol. 2022;5:273–82.
Pienta KJ, Gorin MA, Rowe SP, Carroll PR, Pouliot F, Probst S, et al. A phase 2/3 prospective Multicenter Study of the Diagnostic Accuracy of Prostate Specific Membrane Antigen PET/CT with 18F-DCFPyL in prostate Cancer patients (OSPREY). J Urol. 2021;206:52–61.
Hammes J, Hohberg M, Täger P, Wild M, Zlatopolskiy B, Krapf P, et al. Uptake in non-affected bone tissue does not differ between [18F]-DCFPyL and [68Ga]-HBED-CC PSMA PET/CT. PLoS ONE. 2018;13:e0209613.
Maisto C, Aurilio M, Morisco A, de Marino R, Buonanno Recchimuzzo MJ, Carideo L, et al. Analysis of pros and cons in using [68Ga]Ga-PSMA-11 and [18F]PSMA-1007: production, costs, and PET/CT applications in patients with prostate Cancer. Molecules. 2022;27:3862.
Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–5.
Wollenweber SD, Delso G, Deller T, Goldhaber D, Hüllner M, Veit-Haibach P. Characterization of the impact to PET quantification and image quality of an anterior array surface coil for PET/MR imaging. MAGMA. 2014;27:149–59.
Moazemi S, Khurshid Z, Erle A, Lütje S, Essler M, Schultz T, et al. Machine learning facilitates hotspot classification in PSMA-PET/CT with Nuclear Medicine specialist accuracy. Diagnostics (Basel). 2020;10:622.
Sundahl N, Gillessen S, Sweeney C, Ost P. When what you see is not always what you get: raising the bar of evidence for New Diagnostic Imaging modalities. Eur Urol. 2021;79:565–7.
Bauckneht M, Checcucci E, Cisero E, Rizzo A, Racca M, De Cillis S, et al. The prognostic role of next-generation imaging-driven upstaging in newly diagnosed prostate cancer patients. Eur J Nucl Med Mol Imaging. 2024;51:864–70.
Wang C, Shen Y. Study on the distribution features of bone metastases in prostate cancer. Nucl Med Commun. 2012;33:379–83.
Funding
None.
Open access funding provided by Università degli Studi di Genova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
M.B., A.R., and S.M. contributed to the study’s conception and design. Data collection and analysis were performed by A.R., M.B., D.A., G.F., R.L., M.C., F.D., S.G., F.B., M.R., and G.T. A.R. and M.B. wrote the first draft of the manuscript, and all authors commented on previous versions of the manuscript. S.M. and G.T. revised the final draft critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
N.A.
Competing interests
S.M. received speaker honoraria from GE Healthcare, Life Molecular Imaging, and Eli Lilly. M.B. reports personal fees from AAA and General Electric Healthcare. The other authors do not report any conflict of interest.
Consent to participate
N.A.
Consent for publication
N.A.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rizzo, A., Morbelli, S., Albano, D. et al. The Homunculus of unspecific bone uptakes associated with PSMA-targeted tracers: a systematic review-based definition. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06797-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06797-5