Abstract
Background
Amino acid PET is recommended for the initial diagnosis of brain lesions, but its value for identifying aggressive lesions remains to be established. The current study therefore evaluates the added-value of dynamic [18 F]FDOPA PET as an adjunct to conventional MRI for determining the aggressiveness of presumed glial lesions at diagnosis.
Methods
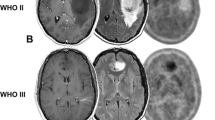
Consecutive patients, with a minimal 1 year-follow-up, underwent contrast-enhanced MRI (CE MRI) and dynamic [18 F]FDOPA PET to characterize their suspected glial lesion. Lesions were classified semi-automatically by their CE MRI (MRI-/+), and PET parameters (static tumor-to-background ratio, TBR; dynamic time-to-peak ratio, TTPratio). Diagnostic accuracies of MRI and PET parameters for the differentiation of tumor aggressiveness were evaluated by chi-square test or receiver operating characteristic analyses. Aggressive lesions were either defined as lesions with dismal molecular characteristics based on the WHO 2021 classification of brain tumors or with compatible clinico-radiological profiles. Time-to-treatment failure (TTF) and overall survival (OS) were evaluated.
Results
Of the 109 patients included, 46 had aggressive lesions (45 confirmed by histo-molecular analyses). CE MRI identified aggressive lesions with an accuracy of 73%. TBRmax (threshold of 3.2), and TTPratio (threshold of 5.4 min) respectively identified aggressive lesions with an accuracy of 83% and 76% and were independent of CE MRI and clinical factors in the multivariate analysis. Among the MRI-lesions, 11/56 (20%) were aggressive and respectively 55% and 50% of these aggressive lesions showed high TBRmax and short TTPratio in PET. High TBRmax and short TTPratio in PET were significantly associated to poorer survivals (p ≤ 0.009).
Conclusion
Dynamic [18 F]FDOPA PET provides a similar diagnostic accuracy as contrast enhancement in MRI to identify the aggressiveness of suspected glial lesions at diagnosis. Both methods, however, are complementary and [18 F]FDOPA PET may be a useful additional tool in equivocal cases.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available on a Supplemental file.
Code Availability
Not applicable.
References
Ellingson BM, Wen PY, Cloughesy TF. Modified Criteria for Radiographic Response Assessment in Glioblastoma clinical trials. Neurotherapeutics. 2017;14:307–20.
Leao DJ, Craig PG, Godoy LF, Leite CC, Policeni B. Response Assessment in Neuro-Oncology Criteria for Gliomas: practical Approach using Conventional and Advanced techniques. AJNR Am J Neuroradiol. 2020;41:10–20.
Albert NL, Weller M, Suchorska B, Galldiks N, Soffietti R, Kim MM, et al. Response Assessment in Neuro-Oncology working group and European Association for Neuro-Oncology recommendations for the clinical use of PET imaging in gliomas. NEUONC. 2016;18:1199–208.
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20.
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neurooncology. 2021;23:1231–51.
Ginet M, Zaragori T, Marie P-Y, Roch V, Gauchotte G, Rech F, et al. Integration of dynamic parameters in the analysis of 18F-FDopa PET imaging improves the prediction of molecular features of gliomas. Eur J Nucl Med Mol Imaging. 2020;47:1381–90.
Zaragori T, Oster J, Roch V, Hossu G, Chawki MB, Grignon R, et al. 18 F-FDOPA PET for the Noninvasive prediction of Glioma Molecular parameters: a Radiomics Study. J Nucl Med. 2022;63:147–57.
Verger A, Metellus Ph, Sala Q, Colin C, Bialecki E, Taieb D, et al. IDH mutation is paradoxically associated with higher [18F]FDOPA PET uptake in diffuse grade II and grade III gliomas. Eur J Nucl Med Mol Imaging. 2017;44:1306–11.
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, et al. Comparison of a Strategy Favoring Early Surgical Resection vs a strategy favoring Watchful Waiting in Low-Grade Gliomas. JAMA. 2012;308:1881.
Obara T, Blonski M, Brzenczek C, Mézières S, Gaudeau Y, Pouget C, et al. Adult diffuse low-Grade gliomas: 35-Year experience at the Nancy France Neurooncology Unit. Front Oncol. 2020;10:574679.
Janvier L, Olivier P, Blonski M, Morel O, Vignaud J-M, Karcher G, et al. Correlation of SUV-Derived indices with Tumoral aggressiveness of Gliomas in Static [18F]FDOPA PET: use in clinical practice. Clin Nucl Med. 2015;40:e429–35.
Isal S, Gauchotte G, Rech F, Blonski M, Planel S, Chawki MB, et al. A high [18F]FDOPA uptake is associated with a slow growth rate in diffuse Grade II-III gliomas. Br J Radiol. 2018;91:20170803.
Patel CB, Fazzari E, Chakhoyan A, Yao J, Raymond C, Nguyen H, et al. [18F]FDOPA PET and MRI characteristics correlate with degree of malignancy and predict survival in treatment-naïve gliomas: a cross-sectional study. J Neurooncol. 2018;139:399–409.
Xiao J, Jin Y, Nie J, Chen F, Ma X. Diagnostic and grading accuracy of [18F]FDOPA PET and PET/CT in patients with gliomas: a systematic review and meta-analysis. BMC Cancer. 2019;19:767.
Kunz M, Albert NL, Unterrainer M, la Fougere C, Egensperger R, Schüller U, et al. Dynamic 18F-FET PET is a powerful imaging biomarker in gadolinium-negative gliomas. Neurooncology. 2019;21:274–84.
Lohmeier J, Radbruch H, Brenner W, Hamm B, Tietze A, Makowski MR. Predictive IDH genotyping based on the evaluation of spatial metabolic heterogeneity by Compartmental Uptake Characteristics in preoperative glioma using 18 F-FET PET. J Nucl Med. 2023;64:1683–9.
Hajri R, Nicod-Lalonde M, Hottinger AF, Prior JO, Dunet V. Prediction of Glioma Grade and IDH Status using 18F-FET PET/CT dynamic and multiparametric texture analysis. Diagnostics. 2023;13:2604.
Mandonnet E, Delattre J-Y, Tanguy M-L, Swanson KR, Carpentier AF, Duffau H, et al. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann Neurol. 2003;53:524–8.
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18:170–86.
Bros M, Zaragori T, Rech F, Blonski M, Hossu G, Taillandier L, et al. Effects of Carbidopa Premedication on [18F]FDOPA PET Imaging of Glioma: a multiparametric analysis. Cancers. 2021;13:5340.
Tustison NJ, Avants BB, Cook PA, Yuanjie Zheng, Egan A, Yushkevich PA, et al. N4ITK: improved N3 Bias correction. IEEE Trans Med Imaging. 2010;29:1310–20.
Nioche C, Orlhac F, Boughdad S, Reuzé S, Goya-Outi J, Robert C, et al. LIFEx: a freeware for Radiomic feature calculation in Multimodality Imaging to accelerate advances in the characterization of Tumor Heterogeneity. Cancer Res. 2018;78:4786–9.
Unterrainer M, Vettermann F, Brendel M, Holzgreve A, Lifschitz M, Zähringer M, et al. Towards standardization of 18F-FET PET imaging: do we need a consistent method of background activity assessment? EJNMMI Res. 2017;7:48.
Girard A, Le Reste P-J, Metais A, Carsin Nicol B, Chiforeanu DC, Bannier E, et al. Combining 18F-DOPA PET and MRI with perfusion-weighted imaging improves delineation of high-grade subregions in enhancing and non-enhancing gliomas prior treatment: a biopsy-controlled study. J Neurooncol. 2021;155:287–95.
Law I, Albert NL, Arbizu J, Boellaard R, Drzezga A, Galldiks N, et al. Joint EANM/EANO/RANO practice guidelines/SNMMI procedure standards for imaging of gliomas using PET with radiolabelled amino acids and [18F]FDG: version 1.0. Eur J Nucl Med Mol Imaging. 2019;46:540–57.
Floberg JM, Mistretta CA, Weichert JP, Hall LT, Holden JE, Christian BT. Improved kinetic analysis of dynamic PET data with optimized HYPR-LR: improved dynamic PET analysis with HYPR-LR. Med Phys. 2012;39:3319–31.
Ahrari S, Zaragori T, Rozenblum L, Oster J, Imbert L, Kas A, et al. Relevance of dynamic 18F-DOPA PET radiomics for differentiation of high-Grade Glioma Progression from Treatment-related changes. Biomedicines. 2021;9:1924.
Mood C. Logistic regression: why we cannot do what we think we can do, and what we can do about it. Eur Sociol Rev. 2010;26:67–82.
Heinzel A, Dedic D, Galldiks N, Lohmann P, Stoffels G, Filss CP, et al. Two decades of Brain Tumour Imaging with O-(2-[18F]fluoroethyl)-L-tyrosine PET: the Forschungszentrum Jülich Experience. Cancers. 2022;14:3336.
Pafundi DH, Laack NN, Youland RS, Parney IF, Lowe VJ, Giannini C, et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neurooncology. 2013;15:1058–67.
Pöpperl G, Kreth FW, Herms J, Koch W, Mehrkens JH, Gildehaus FJ, et al. Analysis of 18F-FET PET for grading of recurrent gliomas: is evaluation of uptake kinetics superior to standard methods? J Nucl Med. 2006;47:393–403.
Verger A, Imbert L, Zaragori T. Dynamic amino-acid PET in neuro-oncology: a prognostic tool becomes essential. Eur J Nucl Med Mol Imaging. 2021;48:4129–32.
Sawlani V, Patel MD, Davies N, Flintham R, Wesolowski R, Ughratdar I, et al. Multiparametric MRI: practical approach and pictorial review of a useful tool in the evaluation of brain tumours and tumour-like lesions. Insights Imaging. 2020;11:84.
Funding
The authors have nothing to disclose.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the analysis and interpretation of the data (AZ, CP, SA, TZ, AV), to the writing of the manuscript (AZ, AV) and to the revision of the manuscript (FR, LT, MB, LI, TZ, AV).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committees and with the 1964 Helsinki declaration and its latest amendments or comparable ethics standards. The institutional ethics committee (Comité d’Ethique du CHRU de Nancy) approved the evaluation of the retrospective patient data and the trial was registered at ClinicalTrials.gov (NCT04469244). The research complied with the principles of the Declaration of Helsinki. Informed consent was obtained from all individuals included in the study.
Consent for publication
Not applicable.
Conflicts of interest/competing interests
The authors disclose no potential conflicts of interest related to the present work.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zinsz, A., Pouget, C., Rech, F. et al. The role of [18 F]FDOPA PET as an adjunct to conventional MRI in the diagnosis of aggressive glial lesions. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06720-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06720-y