Abstract
Purpose
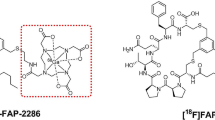
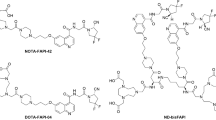
Fibroblast activation protein inhibitor (FAPI) -based probes have been widely studied in the diagnosis of various malignant tumors with positron emission tomography/computed tomography (PET/CT). However, current imaging studies of FAPI-based probes face challenges in rapid clearance rate and potential false-negative results. Furthermore, FAPI has been rarely explored in optical imaging. Considering this, further modifications are imperative to improve the properties of FAPI-based probes to address existing limitations and broaden their application scenarios. In this study, we rationally introduced methylene blue (MB) to FAPIs, thereby imparting nuclei-targeting and fluorescence imaging capabilities to the probes. Furthermore, we evaluated the added value of FAPI-based fluorescence imaging to traditional PET/CT, exploring the potential application of FAPI-based probes in intraoperative fluorescence imaging.
Methods
A new FAPI-based probe, namely NOTA-FAPI-MB, was designed for both PET/CT and fluorescence imaging by conjugation of MB. The targeting efficacy of the probe was evaluated on fibroblast activation protein (FAP)-transfected cell line and human primary cancer-associated fibroblasts (CAFs). Subsequently, PET/CT and fluorescence imaging were conducted on tumor-bearing mice. The tumor detection and boundary delineation were assessed by fluorescence imaging of tissues from hepatocellular carcinoma (HCC) patients.
Results
NOTA-FAPI-MB demonstrated exceptional targeting ability towards FAP-transfected cells and CAFs in comparison to NOTA-FAPI. This benefit arises from the cationic methylene blue (MB) affinity for anionic nucleic acids. PET/CT imaging of tumor-bearing mice revealed significantly higher tumor uptake of [18F]F-NOTA-FAPI-MB (standard uptake value of 2.20 ± 0.31) compared to [18F]F-FDG (standard uptake value of 1.66 ± 0.14). In vivo fluorescence imaging indicated prolonged retention at the tumor site, with retention lasting up to 24 h. In addition, the fluorescent probes enabled more precise lesion detection and tumor margin delineation than clinically used indocyanine green (ICG), achieving a 100.0% (6/6) tumor-positive rate for NOTA-FAPI-MB while 33.3% (2/6) for ICG. These findings highlighted the potential of NOTA-FAPI-MB in guiding intraoperative surgical procedures.
Conclusions
The NOTA-FAPI-MB was successfully synthesized, in which FAPI and MB simultaneously contributed to the targeting effect. Notably, the nuclear delivery mechanism of the probes improved intracellular retention time and targeting efficacy, broadening the imaging time window for fluorescence imaging. In vivo PET/CT demonstrated favorable performance of NOTA-FAPI-MB compared to [18F]F-FDG. This study highlights the significance of fluorescence imaging as an adjunct technique to PET/CT. Furthermore, the encouraging results obtained from the imaging of human HCC tissues hold promise for the potential application of NOTA-FAPI-MB in intraoperative fluorescent surgery guidance within clinical settings.





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. https://doi.org/10.3322/caac.21660.
Soerjomataram I, Bray F. Planning for tomorrow: global cancer incidence and the role of prevention 2020–2070. Nat Rev Clin Oncol. 2021;18:663–72. https://doi.org/10.1038/s41571-021-00514-z.
Miller KD, Nogueira L, Devasia T, Mariotto AB, Yabroff KR, Jemal A, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72:409–36. https://doi.org/10.3322/caac.21731.
Crosby D, Bhatia S, Brindle KM, Coussens LM, Dive C, Emberton M, et al. Early detection of cancer. Science. 2022;375:eaay9040. https://doi.org/10.1126/science.aay9040.
Min JH, Kim JM, Kim YK, Cha DI, Kang TW, Kim H, et al. Magnetic resonance imaging with Extracellular contrast detects Hepatocellular Carcinoma with Greater Accuracy Than with Gadoxetic Acid or computed tomography. Clin Gastroenterol Hepatol. 2020;18. https://doi.org/10.1016/j.cgh.2019.12.010. 2091 – 100 e7.
Balata H, Fong KM, Hendriks LE, Lam S, Ostroff JS, Peled N, et al. Prevention and early detection for NSCLC: advances in thoracic oncology 2018. J Thorac Oncol. 2019;14:1513–27. https://doi.org/10.1016/j.jtho.2019.06.011.
Singhi AD, Koay EJ, Chari ST, Maitra A. Early detection of pancreatic Cancer: opportunities and challenges. Gastroenterology. 2019;156:2024–40. https://doi.org/10.1053/j.gastro.2019.01.259.
Lv J, Xu Y, Xu L, Nie L. Quantitative functional evaluation of liver fibrosis in mice with dynamic contrast-enhanced photoacoustic imaging. Radiology. 2021;300:89–97. https://doi.org/10.1148/radiol.2021204134.
Chen R, Huang S, Lin T, Ma H, Shan W, Duan F, et al. Photoacoustic molecular imaging-escorted adipose photodynamic–browning synergy for fighting obesity with virus-like complexes. Nat Nanotechnol. 2021;16:455–65. https://doi.org/10.1038/s41565-020-00844-6.
Wan H, Du H, Wang F, Dai H. Molecular imaging in the second near-infrared window. Adv Funct Mater. 2019;29. https://doi.org/10.1002/adfm.201900566.
Sollini M, Kirienko M, Gelardi F, Fiz F, Gozzi N, Chiti A. State-of-the-art of FAPI-PET imaging: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2021;48:4396–414. https://doi.org/10.1007/s00259-021-05475-0.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32. https://doi.org/10.1007/s00259-020-04769-z.
Wei Y, Ma L, Li P, Lu J, Ren J, Yan S, et al. FAPI compared with FDG PET/CT for diagnosis of primary and metastatic Lung Cancer. Radiology. 2023;308. https://doi.org/10.1148/radiol.222785.
Jiang D, Wei W. Molecular imaging for better theranostics. Eur J Nucl Med Mol Imaging. 2023;50:3799–801. https://doi.org/10.1007/s00259-023-06415-w.
Altmann A, Haberkorn U, Siveke J. The latest developments in imaging of fibroblast activation protein. J Nucl Med. 2021;62:160–7. https://doi.org/10.2967/jnumed.120.244806.
Shi X, Xing H, Yang X, Li F, Yao S, Congwei J, et al. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: a prospective pilot study. Eur J Nucl Med Mol Imaging. 2021;48:1593–603. https://doi.org/10.1007/s00259-020-05070-9.
Yang T, Peng L, Qiu J, He X, Zhang D, Wu R, et al. A radiohybrid theranostics ligand labeled with fluorine-18 and lutetium-177 for fibroblast activation protein-targeted imaging and radionuclide therapy. Eur J Nucl Med Mol Imaging. 2023;50:2331–41. https://doi.org/10.1007/s00259-023-06169-5.
McGrath JM, Stewart GJ. The effects of endotoxin on vascular endothelium. J Exp Med. 1969;129:833–48. https://doi.org/10.1084/jem.129.5.833.
He J, Li C, Ding L, Huang Y, Yin X, Zhang J, et al. Tumor targeting strategies of smart fluorescent nanoparticles and their applications in cancer diagnosis and treatment. Adv Mater. 2019;31:e1902409. https://doi.org/10.1002/adma.201902409.
Su W, Chen C, Wang T, Li X, Liu Y, Wang H, et al. Radionuclide-labeled gold nanoparticles for nuclei-targeting internal radio-immunity therapy. Mater Horizons. 2020;7:1115–25. https://doi.org/10.1039/C9MH01725A.
Yu Q, Huang S, Wu Z, Zhen J, Chen X, Nie L. Label-free visualization of early Cancer hepatic micrometastasis and intraoperative image-guided surgery by photoacoustic imaging. J Nucl Med. 2020;61:1079. https://doi.org/10.2967/jnumed.119.233155.
Giesel FL, Kratochwil C, Schlittenhardt J, Dendl K, Eiber M, Staudinger F, et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur J Nucl Med Mol Imaging. 2021;48:4377–85. https://doi.org/10.1007/s00259-021-05307-1.
Kudo S-e, Misawa M, Mori Y, Hotta K, Ohtsuka K, Ikematsu H, et al. Artificial intelligence-assisted system improves endoscopic identification of colorectal neoplasms. Clin Gastroenterol Hepatol. 2020;18:1874–81. https://doi.org/10.1016/j.cgh.2019.09.009. .e2.
Wang M, Zhang J, Zheng L, Fang H, Zhang Y, Deng H, et al. Ultrasound-guided continuous thoracic paravertebral infusion of methylene blue in the treatment of postherpetic neuralgia: a prospective, randomized, controlled study. Pain Ther. 2021;10:675–89. https://doi.org/10.1007/s40122-021-00265-w.
Dip F, Boni L, Bouvet M, Carus T, Diana M, Falco J et al. Consensus conference statement on the general use of near-infrared fluorescence imaging and indocyanine green guided surgery: results of a modified delphi study. Ann Surg. 2022;275:685–91. https://doi.org/10.1097/SLA.0000000000004412.
Kwon IG, Son T, Kim H-I, Hyung WJ. Fluorescent lymphography–guided Lymphadenectomy during Robotic Radical Gastrectomy for gastric Cancer. JAMA Surg. 2019;154:150–8. https://doi.org/10.1001/jamasurg.2018.4267.
Zhong Q, Chen QY, Huang XB, Lin GT, Liu ZY, Chen JY, et al. Clinical implications of indocyanine green fluorescence imaging-guided laparoscopic lymphadenectomy for patients with gastric cancer: a cohort study from two randomized, controlled trials using individual patient data. INT J SURG. 2021;94:106120. https://doi.org/10.1016/j.ijsu.2021.106120.
Dendl K, Koerber SA, Finck R, Mokoala KMG, Staudinger F, Schillings L, et al. (68)Ga-FAPI-PET/CT in patients with various gynecological malignancies. Eur J Nucl Med Mol Imaging. 2021;48:4089–100. https://doi.org/10.1007/s00259-021-05378-0.
Wang X, Teh CSC, Ishizawa T, Aoki T, Cavallucci D, Lee S-Y, et al. Consensus guidelines for the use of fluorescence imaging in hepatobiliary surgery. Ann Surg. 2021;274. https://doi.org/10.1097/SLA.0000000000004718.
Zeng X, Zhu W, Lin W, Tian J, Yang J, Fang C. Laparoscopic anatomical extended right posterior sectionectomy using virtual liver segment projection Navigation and Indocyanine Green fluorescence imaging. Ann Surg Oncol. 2023;30:375–6. https://doi.org/10.1245/s10434-022-12551-8.
Zhang J, Mao F, Niu G, Peng L, Lang L, Li F, et al. (68)Ga-BBN-RGD PET/CT for GRPR and integrin alpha(v)beta(3) imaging in patients with breast Cancer. Theranostics. 2018;8:1121–30. https://doi.org/10.7150/thno.22601.
Nammas W, Paunonen C, Teuho J, Siekkinen R, Luoto P, Käkelä M, et al. Imaging of myocardial αvβ3Integrin expression for evaluation of myocardial Injury after Acute myocardial infarction. J Nucl Med. 2024;65:132–8. https://doi.org/10.2967/jnumed.123.266148.
Zhang Y, Lv J, Liu P, Zhao X, Chen K, Li Q, et al. Contrast-enhanced multispectral photoacoustic imaging for irregular hepatectomy navigation: a pilot study. ACS Biomater Sci Eng. 2020;6:5874–85. https://doi.org/10.1021/acsbiomaterials.0c00921.
de Valk KS, Deken MM, Handgraaf HJM, Bhairosingh SS, Bijlstra OD, van Esdonk MJ, et al. First-in-human assessment of cRGD-ZW800-1, a zwitterionic, integrin-targeted, Near-Infrared fluorescent peptide in colon carcinoma. Clin Cancer Res. 2020;26:3990–8. https://doi.org/10.1158/1078-0432.Ccr-19-4156.
Singh G, Gott MD, Pietzsch HJ, Stephan H. Nuclear and optical dual-labelled imaging agents. Design and challenges. Nuklearmedizin. 2016;55:41–50. https://doi.org/10.1055/s-0037-1616472.
Wen X, Xu P, Shi M, Liu J, Zeng X, Zhang Y, et al. Evans blue-modified radiolabeled fibroblast activation protein inhibitor as long-acting cancer therapeutics. Theranostics. 2022;12:422–33. https://doi.org/10.7150/thno.68182.
Funding
This work was supported by the National Natural Science Foundation of China (82372010 & 22077061), National Key R&D Program of China (2023YFF0715303), Guangdong Provincial People’s Hospital Supporting Fund for Talent Program (KJ012020638) and Guangdong Provincial Key Laboratory of Tumor Interventional Diagnosis and Treatment (2021B1212040004).
Author information
Authors and Affiliations
Contributions
Liming Nie, Chuangyan Zhai were responsible for the conception and design of the study, critical revision of the manuscript for important intellectual content and final approval of the version to be published. Xingyang Zhao, Guojin Zhang, Zirong Li were responsible for the the acquisition, analysis and interpretation of the data, and the drafting of the manuscript. Jiali Chen assisted the analysis of the data and revision of the manuscripts. Yusheng Shi and Guiting Li assisted the analysis of the data.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
For human tissues, the study was approved by the ethics committee of Guangdong provincial people′s hospital (S2023-1055-01). For animal subjects, all experimental protocols were approved by the ethics committee of Guangdong provincial people′s hospital (2021006).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in the Fig. 5a.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, X., Zhang, G., Chen, J. et al. A rationally designed nuclei-targeting FAPI 04-based molecular probe with enhanced tumor uptake for PET/CT and fluorescence imaging. Eur J Nucl Med Mol Imaging 51, 1593–1604 (2024). https://doi.org/10.1007/s00259-024-06691-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-024-06691-0