Abstract
Purpose
The use of [177Lu]Lu-PSMA-617 radioligand therapy has become increasingly recognized as a viable therapeutic approach for patients in the advanced stages of metastatic castration-resistant prostate cancer (mCRPC). However, there is limited data regarding its effectiveness and safety in earlier lines. This study aims to present our institution’s experience with [177Lu]Lu-PSMA-617 as a first-line systemic therapy for mCRPC.
Methods
We collected and analyzed data from consecutive mCRPC patients who underwent first-line treatment with [177Lu]Lu-PSMA-617 at our center from 2015 to 2023. The various outcome measures included best prostate-specific antigen-response rate (PSA-RR) (proportion of patients achieving a ≥ 50% decline in PSA); objective radiographic response rate (ORR) (proportion of patients achieving complete or partial radiographic responses); radiographic progression-free survival (rPFS) (measured from treatment initiation until radiographic progression or death from any cause); overall survival (OS) (measured from treatment initiation until death from any cause); and adverse events.
Results
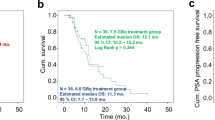
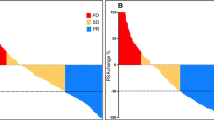
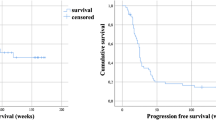
Forty treatment-naïve mCRPC patients with PSMA-positive disease on [68Ga]Ga-PSMA-11 PET/CT were included (median age: 68.5 years, range: 45–78; median PSA: 41 ng/mL, range: 1-3028). These patients received a median cumulative activity of 22.2 GBq (range: 5.55–44.4) [177Lu]Lu-PSMA-617 over 1–6 cycles at 8–12 week intervals. A ≥ 50% decline in PSA was observed in 25/40 (62.5%) patients (best PSA-RR). Radiographic responses were evaluated for thirty-eight patients, with thirteen showing partial responses (ORR 34.2%). Over a median follow-up of 36 months, the median rPFS was 12 months (95% confidence interval, CI: 9–15), and the median OS was 17 months (95% CI: 12–22). Treatment-emergent grade ≥ 3 anemia, leucopenia, and thrombocytopenia were noted in 4/40 (10%), 1/40 (2.5%), and 3/40 (7.5%) patients, respectively.
Conclusion
The findings suggest that [177Lu]Lu-PSMA-617 is a safe and effective option as a first-line treatment in mCRPC. Further trials are needed to definitively establish its role as an upfront treatment modality in this setting.



Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17–48.
Mansinho A, Macedo D, Fernandes I, et al. Castration-resistant prostate Cancer: mechanisms, targets and treatment. Adv Exp Med Biol. 2018;1096:117–33.
Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate Cancer. Part II-2020 update: treatment of relapsing and metastatic prostate Cancer. Eur Urol. 2021;79:263–82.
Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12.
de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005.
Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16:152–60.
Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–97.
Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with Chemotherapy-naïve metastatic castration-resistant prostate Cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–4.
Mateo J, Boysen G, Barbieri CE, et al. DNA repair in prostate Cancer: Biology and Clinical implications. Eur Urol. 2017;71:417–25.
Rose M, Burgess JT, O’Byrne K, et al. PARP inhibitors: clinical relevance, mechanisms of Action and Tumor Resistance. Front Cell Dev Biol. 2020;8:564601.
Tisseverasinghe S, Bahoric B, Anidjar M, et al. Advances in PARP inhibitors for prostate Cancer. Cancers (Basel). 2023;15:1849.
Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone acetate for metastatic castration-resistant prostate Cancer. J Clin Oncol. 2023;41:3339–51.
Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evid. 2022;1:EVIDoa2200043.
Agarwal N, Azad AA, Carles J, et al. Talazoparib plus Enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402:291–303.
Tanaka A, Node K. The Emerging and Promising Role of Care for Cardiometabolic syndrome in prostate Cancer. JACC CardioOncol. 2019;1:307–9.
Yadav MP, Ballal S, Sahoo RK, et al. Radioligand Therapy with 177Lu-PSMA for metastatic castration-resistant prostate Cancer: a systematic review and Meta-analysis. AJR Am J Roentgenol. 2019;213:275–85.
Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397:797–804.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate Cancer. N Engl J Med. 2021;385:1091–103.
Satapathy S, Mittal BR, Sood A, et al. 177Lu-PSMA-617 versus docetaxel in chemotherapy-naïve metastatic castration-resistant prostate cancer: a randomized, controlled, phase 2 non-inferiority trial. Eur J Nucl Med Mol Imaging. 2022;49:1754–64.
Satapathy S, Mittal BR, Sood A, et al. [177Lu]Lu-PSMA-617 Versus Docetaxel in Chemotherapy-Naïve metastatic castration-resistant prostate Cancer: final survival analysis of a phase 2 Randomized, Controlled Trial. J Nucl Med. 2023;64:1726–9.
Sadaghiani MS, Sheikhbahaei S, Werner RA, et al. 177 Lu-PSMA radioligand therapy effectiveness in metastatic castration-resistant prostate cancer: an updated systematic review and meta-analysis. Prostate. 2022;82:826–35.
Scher HI, Morris MJ, Stadler WM, et al. Trial Design and objectives for castration-resistant prostate Cancer: updated recommendations from the prostate Cancer clinical trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–47.
Sartor O, Castellano Gauna DE, Herrmann K, et al. LBA13 phase III trial of [177Lu]Lu-PSMA-617 in taxane-naive patients with metastatic castration-resistant prostate cancer (PSMAfore). Ann Oncol. 2023;34(Suppl 2):S1324–5.
Satapathy S, Das N, Sood A, et al. Short-course 177Lu-PSMA-617 Radioligand Therapy in High-volume metastatic hormone-sensitive prostate Cancer: time to take the Leap? Eur Urol. 2021;80:390–2.
Privé BM, Peters SMB, Muselaers CHJ, et al. Lutetium-177-PSMA-617 in low-volume hormone-sensitive metastatic prostate Cancer: a prospective pilot study. Clin Cancer Res. 2021;27:3595–601.
Shore ND, Laliberté F, Ionescu-Ittu R, et al. Real-world treatment patterns and overall survival of patients with metastatic castration-resistant prostate Cancer in the US prior to PARP inhibitors. Adv Ther. 2021;38:4520–40.
Galli L, Chiuri VE, Di Lorenzo G, et al. First-line treatment of metastatic castration-resistant prostate cancer: the real-world Italian cohort of the prostate Cancer Registry. Tumori. 2023;109:224–32.
Shayegan B, Wallis CJD, Malone S, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol. 2022;40:e1921–9.
Anton A, Pillai S, Semira MC, et al. Real-world first-line systemic therapy patterns in metastatic castration-resistant prostate cancer. BJUI Compass. 2021;3:205–13.
Chowdhury S, Bjartell A, Lumen N, et al. Real-world outcomes in First-Line treatment of metastatic castration-resistant prostate Cancer: the prostate Cancer Registry. Target Oncol. 2020;15:301–15.
Bjartell A, Lumen N, Maroto P, et al. Real-world safety and efficacy outcomes with abiraterone acetate plus prednisone or prednisolone as the First- or second-line treatment for metastatic castration-resistant prostate Cancer: data from the prostate Cancer Registry. Target Oncol. 2021;16:357–67.
Freedland SJ, Davis M, Epstein AJ et al. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023 Oct 2. https://doi.org/10.1038/s41391-023-00725-8. Epub ahead of print.
George DJ, Sartor O, Miller K, et al. Treatment patterns and outcomes in patients with metastatic castration-resistant prostate Cancer in a real-world clinical practice setting in the United States. Clin Genitourin Cancer. 2020;18:284–94.
Satapathy S, Sahoo RK, Bal C. [177Lu]Lu-PSMA-Radioligand Therapy Efficacy outcomes in Taxane-Naïve Versus taxane-treated patients with metastatic castration-resistant prostate Cancer: a systematic review and metaanalysis. J Nucl Med. 2023;64:1266–71.
Privé BM, Slootbeek PHJ, Laarhuis BI, et al. Impact of DNA damage repair defects on response to PSMA radioligand therapy in metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2022;25:71–8.
Satapathy S, Das CK, Aggarwal P, et al. Genomic characterization of metastatic castration-resistant prostate cancer patients undergoing PSMA radioligand therapy: a single-center experience. Prostate. 2023;83:169–78.
Satapathy S, Mittal BR, Sood A. Visceral metastases as predictors of response and survival outcomes in patients of castration-resistant prostate Cancer treated with 177Lu-Labeled prostate-specific membrane Antigen Radioligand Therapy: a systematic review and Meta-analysis. Clin Nucl Med. 2020;45:935–42.
Mehrens D, Kramer KKM, Unterrainer LM, et al. Cost-effectiveness analysis of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castration-resistant prostate Cancer. J Natl Compr Canc Netw. 2023;21:43–e502.
Gong CL, Hay JW. Cost-effectiveness analysis of abiraterone and sipuleucel-T in asymptomatic metastatic castration-resistant prostate cancer. J Natl Compr Canc Netw. 2014;12:1417–25.
Acknowledgements
None.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Drs. Satapathy and Bal conceived the idea; Drs. Satapathy, Yadav, Ballal, and Sahoo collected the data; Drs. Satapathy, and Ballal analyzed the data; Dr. Satapathy wrote the initial draft of the manuscript; Drs. Yadav and Ballal modified the manuscript; Drs. Sahoo and Bal critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institute Ethics Committee, All India Institute of Medical Sciences, New Delhi (Ref. No. IESC/T-229/05.05.2015, RT-46/2015).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Satapathy, S., Yadav, M., Ballal, S. et al. [177Lu]Lu-PSMA-617 as first-line systemic therapy in patients with metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging (2024). https://doi.org/10.1007/s00259-024-06677-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00259-024-06677-y