Abstract
Purpose
We evaluated the impact on cardiovascular outcome of coronary revascularization-induced changes in ischemic total perfusion defect (ITPD) and myocardial flow reserve (MFR) as assessed by 82Rb positron emission tomography (PET)/computed tomography (CT) imaging.
Methods
The study included 102 patients referred to 82Rb PET/CT myocardial perfusion imaging before and after coronary revascularization. All patients were followed for the occurrence of cardiovascular events (cardiac death, nonfatal myocardial infarction, repeated revascularization, and heart failure) after the second imaging study.
Results
During a median follow-up of 20 months, 21 events occurred. The clinical characteristics were comparable between patients with and without events. In the overall study population, after revascularization, there was a significant reduction (P < 0.001) of ITPD, while hyperemic myocardial blood flow (MBF) (P < 0.01) and MFR (P < 0.05) significantly improved. Event rate was higher in patients with ITPD (P < 0.005) or MFR (P < 0.001) worsening compared to those with unchanged or improved ITPD or MFR. At Cox univariable analysis, ITPD and MFR worsening resulted in predictors of events (both P < 0.05). Patients with worsening of both ITPD and MFR had the worst event-free survival (log-rank 32.9, P for trend < 0.001).
Conclusions
In patients with stable CAD, worsening of ITPD and MFR after revascularization procedures is associated with higher risk of cardiovascular events. Follow-up MPI with 82Rb PET/CT may improve risk stratification in patients submitted to coronary revascularization.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiac imaging by positron emission tomography (PET)/computed tomography (CT) represents a useful noninvasive method for the evaluation of myocardial perfusion and coronary vascular function [1]. The quantification of PET/CT data provides an integrated measurement of myocardial blood flow (MBF), myocardial flow reserve (MFR), and coronary artery calcium content. Therefore, PET/CT is considered a powerful tool in advance current cardiovascular practice in guiding revascularization decisions, potentially for optimal outcomes [2]. The recent European Society of Cardiology guidelines outlined that coronary revascularization has the primary role to improve myocardial perfusion with reducing ischemia [3]. The benefit of coronary revascularization is closely related to the extent of ischemia reduction [4, 5]; however, the clinical impact of revascularization in patient with stable coronary artery disease (CAD) remains to be fully addressed. It has been demonstrated that reduced MFR by PET myocardial perfusion imaging (MPI) is associated with adverse cardiovascular events and patients with low MFR appeared to benefit most from coronary revascularization [6]. However, few studies evaluated the impact on cardiovascular outcome of the changes in myocardial perfusion and absolute MBF after revascularization procedures [7, 8]. Recently, it has been showed that hyperemic MBF and MFR by serial [15O]H2O PET may identify patients in whom revascularization will restore myocardial perfusion, potentially improving prognosis [9, 10]. Moreover, 82Rb PET demonstrated good repeatability in the serial evaluation of rest and hyperemic MBF measurements [11], supporting an optimal use in quantifying the possible effects of therapeutic interventions on both myocardial ischemia and MBF. The aim of this study was to evaluate the impact on cardiovascular outcome of coronary revascularization-induced changes in ischemic total perfusion defect (ITPD) and MFR as assessed by 82Rb PET imaging.
Methods
Patients
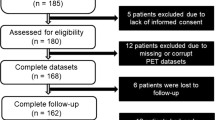
This retrospective study included 120 consecutive patients with suspected or known CAD who underwent stress-rest 82Rb PET/CT at baseline (MPI-1) and at follow-up (MPI-2), after clinically driven coronary revascularization (percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG)). The clinical indication for revascularization was performed by referring physician based on angina symptoms, evaluation of stress-induced ischemia, viability testing when available, and invasive coronary angiography. Patients were referred for MPI after revascularization if they were symptomatic. Asymptomatic patients were referred due to incomplete or suboptimal revascularization or as part of risk stratification [12]. The mean interval time between revascularization and MPI-2 was 15 ± 9 months. No cardiac events occurred between coronary revascularization and MPI-2. From the initial cohort of 120 patients, those with previous CABG, left ventricular ejection fraction < 40%, or clinical diagnosis of heart failure before MPI-1 were excluded, leaving a final cohort of 102 patients. As part of the baseline examination, clinical teams collected information on traditional cardiovascular risk factors (including age, sex, body mass index, diabetes, dyslipidemia, smoking, hypertension, and family history of CAD). Patients were classified as having diabetes if they were receiving treatment with oral hypoglycemic drugs or insulin. Hypertension was defined as a blood pressure ≥ 140/90 mm Hg or the use of anti-hypertensive medication [13]. Hypercholesterolemia was defined as total cholesterol level ≥ 6.2 mmol/L or treatment with cholesterol lowering medication. A positive family history of CAD was defined by the presence of disease in first-degree relatives younger than 55 years in men or 65 years in women. Angina symptoms were defined in the presence of typical angina, atypical angina, or non-anginal chest pain [3]. The review committee of our institution approved the study, and all patients gave informed consent (Ethics Committee, University Federico II, protocol number 110/17).
PET imaging
As a routine preparation for 82Rb cardiac PET/CT, patients were asked to discontinue taking nitrates for 6 h, calcium channel blockers and caffeine-containing beverages for 24 h, and beta-blockers for 48 h before their appointment. Scans were acquired using a Biograph mCT 64-slice scanner (Siemens Healthcare). For both rest and stress images, 1110 MBq of 82Rb was injected intravenously, and a 6-min list-mode PET study was acquired. Pharmacologic stress was then administered using adenosine (140 μg × kg−1 × min−1 for 4.5 min). Both rest and stress dynamic images were reconstructed into 26 time frames (12 × 5 s, 6 × 10 s, 4 × 20 s, and 4 × 40 s; total, 6 min) using the vendor standard ordered subset expectation maximization 3D reconstruction (2 iterations, 24 subsets) with 6.5-mm Gaussian post-processing filter. The images were corrected for attenuation using the low-dose CT. Hemodynamic parameters and 12-lead ECG were recorded at baseline and throughout the infusion of adenosine. Trans-axial PET perfusion images were automatically reoriented into short-axis and vertical and horizontal long-axis slices. Regional myocardial perfusion was visually assessed, using standardized segmentation of 17 myocardial regions [14]. Total perfusion defect (TPD) reflecting a combination of both severity and extent of myocardial defect was calculated using automated software (Cedars-Sinai Medical Center, Los Angeles, CA) [15]. The ischemic TPD (ITPD) was defined as stress TPD − rest TPD and expressed as % of left ventricle. A change of ≥ 5% was used as the criterion for a significant serial change in ITPD in an individual patient [16]. The variations in perfusion pattern between MPI-1 and MPI-2 were categorized as improvement when there was a decrease in ITPD value ≥ 5%; no change; and worsening with an increase of ≥ 5% in ITPD.
Absolute MBF (in ml/min/g) was computed from the dynamic rest and stress imaging series with commercially available software (FlowQuant, University of Ottawa Heart Institute) [17]. MFR was defined as the ratio of hyperemic to baseline MBF and was considered reduced when < 2 [18]. Variations in quantitative pattern were categorized as improvement with recovery of MFR form reduced values to normal values after revascularization; no change; and worsening with change in MFR from normal values to reduced values after revascularization.
Follow-up data
Patient follow-up was prospectively obtained by the use of a questionnaire that was assessed by a phone call to all patients and general practitioners or cardiologists and by review of hospital or physicians’ records by individuals blinded to the patient’s test results. For the purpose of the present investigation, we performed a landmark analysis starting follow-up from MPI-2 [19]. The outcome was a composite endpoint of cardiovascular events including cardiac death, nonfatal myocardial infarction, repeated revascularization, and heart failure, whichever came first. The cause of death was confirmed by review of death certificate, hospital chart, or physician’s records. Death was considered of cardiac origin if the primary cause was defined as acute myocardial infarction, congestive heart failure, valvular heart disease, sudden cardiac death, or cardiac interventional/ surgical procedure related. Myocardial infarction was defined when > 2 of the following 3 criteria were met: chest pain or equivalent symptom complex, positive cardiac biomarkers, or typical electrocardiographic changes [20]. The date of the last examination or consultation was used to determine the length of follow-up.
Statistical analysis
Continuous data are expressed as mean ± standard deviation and categorical data as percentage. A student two-sample t-test and chi-square test were used to compare the differences in continuous and categorical variables, respectively. A P < 0.05 (two-sided) was considered statistically significant. Annualized event rates (AER), expressed as % person-years, were calculated as the cumulative number of events divided by person-time. This latter is an estimate of the actual time at risk that all persons contribute to the study, i.e., the sum of each individual follow-up period. Hazard ratios with 95% confidence intervals (CI) were calculated by univariable Cox regression analysis. The incremental prognostic value of clinical data and imaging findings considering variables in hierarchical order was assessed by the likelihood ratio χ2. Event-free survival curves were obtained by the Kaplan–Meier method and compared with the log-rank test. Statistical analysis was performed with Stata 12 software (StataCorp, College Station, TX, USA).
Results
No patients were lost at follow-up. During a median follow-up of 20 months from MPI-2 (range 7–67 months), 21 events occurred (21% cumulative event rate, annual event rate 9.8% person-years). The events were cardiac death in 2 (10%) patients, nonfatal myocardial infarction in 4 (16%), repeated revascularization in 14 (68%), and heart failure in 1 (5%) patient. Baseline clinical characteristics were comparable between patients with and without events (Table 1).
Imaging findings at MPI-1 and MPI-2 in the overall patient population are reported in Table 2. The scatterplots of MPI-1 and MPI-2 perfusion findings in patients with and without events are reported in Fig. 1. Mean values of hyperemic MBF, MFR, and ITPD were comparable between MPI-1 and MPI-2 in patients with events, while MBF and MFR were higher and ITPD lower on MPI-2 compared with MPI-1 in patients without events (Table 3).
Changes in perfusion findings according to events
ITPD improved in 43 (42%) patients, remained unchanged in 45 (44%), and worsened in 14 (14%) patients. MFR improved in 26 (25%) patients, remained unchanged in 60 (59%), and worsened in 16 (16%) patients. At logistic regression analysis, ITPD and MFR at MPI-1 resulted as predictors of ITPD and MFR improvement, respectively (both P < 0.01).
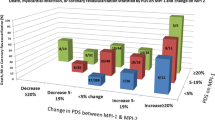
Cardiac events were 3 in patients with improved ITPD, 9 in patients with unchanged, and 9 in those with worsened ITPD. The AER was higher (40%) in patients with ITPD worsening compared to those with unchanged (9%) or improved ITPD (3%) (P for trend < 0.005).
Patients with improved MFR had no events. Cardiac events were 11 in patients with unchanged and 10 in those with worsened MFR. Accordingly, the AER was higher in patients with worsened MFR (34%) compared to those with unchanged (8%) or improved MFR (0%) (P for trend < 0.001).
Predictors of events
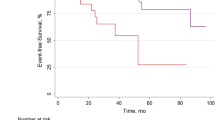
At Cox univariable analysis both ITPD and MFR worsening resulted as predictors of events (Table 4). The AER of patients with or without ITPD and MFR worsening is reported in Fig. 2. As showed, patients with ITPD worsening showed a better outcome in the presence of improved or no change MFR compared to patients with both ITPD and MFR worsening (P < 0.001). Patients with improvement or no change of ITPD showed a worst outcome in the presence of MFR worsening (P < 0.05). The worst outcome was observed in patients with ITPD and MFR worsening (log-rank 32.9, P for trend < 0.001) (Fig. 3). At incremental analysis (Fig. 4), the addition of ITPD worsening to a model including only clinical data increased the global χ2 from 4.26 to 24.92 (P < 0.001). The addition of MFR worsening to a model including clinical data and ITPD further increased the χ2 to 35.06 (P < 0.05).
Discussion
The present study evaluated the impact of change in myocardial perfusion, MBF, and MFR after coronary revascularization in patients undergoing MPI by 82Rb PET/CT. We found that the combined worsening of MFR and ITPD after revascularization was the strongest predictor of poor outcome, associated with a higher risk of events during follow-up.
The quantification of absolute MBF and MFR by PET as markers of coronary vascular function provides an incremental value compared to clinical and perfusion data for both diagnostic and prognostic purposes in patients with suspected or known CAD [21,22,23]. Previous studies pointed up on prognostic value of MFR [24,25,26,27], demonstrating that the coronary vasodilator dysfunction is a powerful and independent correlate of cardiac mortality and provides significant incremental risk stratification [28,29,30]. The presence of reduced cardiac vascular function was associated with an increased risk of cardiac death even in the presence of normal scans by semi-quantitative visual analysis and independently of other risk factors [31,32,33,34]. Myocardial perfusion evaluated by both single-photon emission computed tomography [SPECT] and PET imaging which has been widely used in the follow-up period after revascularization procedure, aiming for guiding the clinical decision-making process and/or to determine the time to retest [34]. It has demonstrated that clinical variables and the presence of myocardial ischemia in post-revascularization patients are useful to predict cardiac events during long-term follow-up [34, 35]. Moreover, both the degree of stress-induced ischemia and left ventricular function can predict the effect of revascularization on outcome in patients with suspected or known CAD [36].
The availability of software for automated reproducible assessment of MPI makes effective the quantitative evaluation of perfusion parameters in serial evaluation [37, 38]. However, few data tested the impact of myocardial perfusion changes in patients on treatment for stable CAD. Moreover, only few data evaluated the impact of changes of myocardial perfusion after revascularization treatment at follow-up.
The prognostic significance of ischemia reduction was examined in a small subgroup of patients, in the adenosine sestamibi SPECT post-infarction evaluation (INSPIRE) study [39]. Ischemia reduction resulted univariate predictor of major adverse cardiac events [40]. In a larger report series, Farzaneh-Far et al. [40] found that 5% worsening ischemia, after medical or revascularization therapy, was a strong predictor of death or myocardial infarction.
As well, the prognostic value of MFR changes specifically after invasive treatment has been poorly investigated. Although the benefit of coronary revascularization is closely related on the severity of baseline myocardial perfusion defects and the extent of ischemia reduction, it has been recently demonstrated that the coronary flow capacity, which combines hyperemic MBF and MFR using [15O]H2O PET perfusion imaging, represents a diagnostic tool associated with outcome after revascularization therapy [10]. In particular, in patients with a significant amount of ischemic myocardium, the recovery of vasodilator capacity could help to identify those that will benefit most from revascularization. On the contrary, patients in whom the revascularization procedure is not accompanied by an increase in MBF may have a poor outcome.
In our study, all patients underwent 82Rb PET/CT for the evaluation of cardiac vascular function and myocardial perfusion. Limited data are available on the value of 82Rb PET/CT before and after revascularization procedure, despite MBF by 82Rb demonstrated high reproducibility using a same day short-term repeatability protocol [11]. The reproducibility of 82Rb MBF assessment is important for serial PET measurements after various therapeutic strategies [11]. In addition, cardiac imaging with 82Rb PET/CT is able to provide an accurate measurement of atherosclerotic burden, myocardial perfusion, and coronary vascular function in one examination [41], with a better risk stratification of patient with CAD and prediction of the presence of obstructive CAD [42, 43].
In agreement with a prior study [9], we found that in patients with stable CAD, coronary revascularization was associated with an improvement of hyperemic MBF, MFR, and ITPD. In particular, when we classified patients according to the occurrence of events, those without events showed both a reduction of myocardial ischemic burden and an improvement of coronary blood flow, not detectable in the group of patients with events.
We also found that worsening of ischemic burden as well as of MFR resulted in predictors of cardiac events during the follow-up after coronary revascularization. Not surprisingly, the worst outcome was present in patients with worsening of both parameters. Patients with ITPD worsening showed a better outcome in the presence of an improved or no change MFR compared to patients with both ITPD and MFR worsening. Moreover, patients with improvement or no change of ITPD showed a worst outcome in the presence of MFR worsening. In detail, how impaired MFR is associated with increased clinical risk and precisely how it could modify the effect of revascularization cannot be determined from this study, although from our data it seems that MFR change represents a significant prognostic factor. In our study, population revascularization improved measures of myocardial perfusion in 42% of patients. The presence of residual perfusion abnormalities may be due to incompleteness or failed revascularization procedures or to disease progression potentially associated with limited survival due to residual diffuse CAD. These mechanisms most likely represent the basis for failure of randomized trials to improve survival after revascularization in stable CAD [44].
Our preliminary results involved a limited number of patients in a short-term follow-up. Probably an analysis in a larger study population followed for a longer follow-up time could help to better elucidate the prognostic value of changes in cardiac vascular function after revascularization and clarify if the changes of myocardial flow could be independent by the ischemic burden.
Conclusions
In patients with stable CAD, the presence of worsening of ITPD and MPR after coronary revascularization procedures was associated with a high risk of cardiovascular events during follow-up. Serial MPI imaging with 82Rb PET/CT may improve risk stratification in patients undergoing coronary revascularization.
References
Ziadi MC. Myocardial flow reserve (MFR) with positron emission tomography (PET)/computed tomography (CT): clinical impact in diagnosis and prognosis. Cardiovasc Diagn Ther. 2017;7:206–18.
Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62:1639–53.
Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–77.
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation [COURAGE] trial nuclear substudy. Circulation. 2008;117:1283–91.
Stone GW, Hochman JS, Williams DO, Boden WE, Ferguson TB Jr, Harrington RA, et al. Medical therapy with versus without revascularization in stable patients with moderate and severe ischemia: the case for community equipoise. J Am Coll Cardiol. 2016;67:81–99.
Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, et al. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation. 2015;131:19–27.
Bober RM, Milani RV, Oktay AA, Javed F, Polin NM, Morin DP. The impact of revascularization on myocardial blood flow as assessed by positron emission tomography. EJNM. 2019;46:1226–39.
Gould KL, Johnson NP, Roby AE, Bui L, Kitkungvan D, Patel MB, et al. Coronary flow capacity and survival prediction after revascularization: physiological basis and clinical implications. Eur Heart J. 2023;ehad579. https://doi.org/10.1093/eurheartj/ehad579.
Driessen RS, Danad I, Stuijfzand WJ, Schumacher SP, Knuuti J, Mäki M, et al. Impact of revascularization on absolute myocardial blood flow as assessed by serial [15O]H2O positron emission tomography imaging: a comparison with fractional flow reserve. Circ Cardiovasc Imaging. 2018;11: e007417.
de Winter RW, Jukema RA, van Diemen PA, Schumacher SP, Driessen RS, Stuijfzand WJ, et al. The impact of coronary revascularization on vessel-specific coronary flow capacity and long-term outcomes: a serial [15O]H2O positron emission tomography perfusion imaging study. Eur Heart J Cardiovasc Imaging. 2022;23:743–52.
Manabe O, Yoshinaga K, Katoh C, Naya M, deKemp RA, Tamaki N. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med. 2009;50:68–71.
Schindler TH, Bateman TM, Berman DS, Chareonthaitawee P, De Blanche LE, Dilsizian V, et al. Appropriate use criteria for PET myocardial perfusion imaging. J Nucl Med. 2020;61:1221–65.
Rosendorff C, Black HR, Cannon CP, Gersh BJ, Gore J, Izzo JL Jr, et al. Treatment of hypertension in the prevention and management of ischemic heart disease: a scientific statement from the American Heart Association Council for High Blood Pressure Research and the Councils on Clinical Cardiology and Epidemiology and Prevention. Circulation. 2007;115:2761–88.
Cerqueira M, Weissman N, Dilsizian V, Jacobs A, Kaul S, Laskey W, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging Committee of the Council on clinical cardiology of the American Heart Association. Circulation. 2002;105:539–42.
Nakazato R, Dey D, Alexánderson E, Meave A, Jiménez M, Romero E, et al. Automatic alignment of myocardial perfusion PET and 64-slice coronary CT angiography on hybrid PET/CT. J Nucl Cardiol. 2012;19:482–91.
Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, et al. COURAGE Investigators. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–91.
Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 PET and a highly automated analysis program. J Nucl Cardiol. 2010;17:600–16. https://doi.org/10.1007/s12350-010-9225-3.
Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830–40.
Morgan CJ. Landmark analysis: a primer. J Nucl Cardiol. 2019;26:391–3.
Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. ESC scientific document group. Fourth universal definition of myocardial infarction. Eur Heart J. 2019;40:237–69.
Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med. 2014;55:248–55. https://doi.org/10.2967/jnumed.113.121442.
Assante R, Zampella E, Arumugam P, Acampa W, Imbriaco M, Tout D, et al. Quantitative relationship between coronary artery calcium and myocardial blood flow by hybrid rubidium-82 PET/CT imaging in patients with suspected coronary artery disease. J Nucl Cardiol. 2017;24:494–501.
Assante R, Acampa W, Zampella E, Arumugam P, Nappi C, Gaudieri V, et al. Prognostic value of atherosclerotic burden and coronary vascular function in patients with suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2017;44:2290–8.
Daniele S, Nappi C, Acampa W, Storto G, Pellegrino T, Ricci F, et al. Incremental prognostic value of coronary flow reserve assessed with single-photon emission computed tomography. J Nucl Cardiol. 2011;18:612–9.
Assante R, Mainolfi CG, Zampella E, Gaudieri V, Nappi C, Mannarino T, et al. Relation between myocardial blood flow and cardiac events in diabetic patients with suspected coronary artery disease and normal myocardial perfusion imaging. J Nucl Cardiol. 2021;28:1222–33.
Gaudieri V, Mannarino T, Zampella E, Assante R, D’Antonio A, Nappi C, et al. Prognostic value of coronary vascular dysfunction assessed by rubidium-82 PET/CT imaging in patients with resistant hypertension without overt coronary artery disease. Eur J Nucl Med Mol Imaging. 2021;48:3162–71.
Zampella E, Mannarino T, D’Antonio A, Assante R, Gaudieri V, Buongiorno P, et al. Prediction of outcome by 82Rb PET/CT in patients with ischemia and nonobstructive coronary arteries. J Nucl Cardiol. 2023;30:1110–7.
von Felten E, Benz DC, Benetos G, Baehler J, Patriki D, Rampidis GP, et al. Prognostic value of regional myocardial flow reserve derived from 13N-ammonia positron emission tomography in patients with suspected coronary artery disease. Eur J Nucl Med Mol Imaging. 2021;49:311–20.
Green R, Cantoni V, Acampa W, Assante R, Zampella E, Nappi C, et al. Prognostic value of coronary flow reserve in patients with suspected or known coronary artery disease referred to PET myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol. 2021;28:904–18.
Dorbala S, Di Carli MF, Beanlands RS, Merhige ME, Williams BA, Veledar E, et al. Prognostic value of stress myocardial perfusion positron emission tomography: results from a multicenter observational registry. J Am Coll Cardiol. 2013;61:176–84.
Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58:740–8.
Gupta A, Taqueti VR, van de Hoef TP, Bajaj NS, Bravo PE, Murthy VL, et al. Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation. 2017;136:2325–36.
Nappi C, Nicolai E, Daniele S, Acampa W, Gaudieri V, Assante R, et al. Long-term prognostic value of coronary artery calcium scanning, coronary computed tomographic angiography and stress myocardial perfusion imaging in patients with suspected coronary artery disease. J Nucl Cardiol. 2018;25:833–41.
Acampa W, Petretta MP, Daniele S, Perrone-Filardi P, Petretta M, Cuocolo A. Myocardial perfusion imaging after coronary revascularization: a clinical appraisal. Eur J Nucl Med Mol Imaging. 2013;40:1275–82.
Acampa W, Petretta M, Florimonte L, Mattera A, Cuocolo A. Prognostic value of exercise cardiac tomography performed late after percutaneous coronary intervention in symptomatic and symptom-free patients. Am J Cardiol. 2003;91:259–63.
Petretta M, Acampa W, Daniele S, Zampella E, Assante R, Nappi C, et al. Long-term survival benefit of coronary revascularization in patients undergoing stress myocardial perfusion imaging. Circ J. 2016;80:485–93.
Iskandrian AE, Hage FG, Shaw LJ, Mahmarian JJ, Berman DS. Serial myocardial perfusion imaging: defining a significant change and targeting management decisions. JACC Cardiovasc Imaging. 2014;7:79–96.
Berman DS, Kang X, Schisterman EF, Gerlach J, Kavanagh PB, Areeda JS, et al. Serial changes on quantitative myocardial perfusion SPECT in patients undergoing revascularization or conservative therapy. J Nucl Cardiol. 2001;8:428–37.
Mahmarian JJ, Shaw LJ, Olszewski GH, Pounds BK, Frias ME, Pratt CM, INSPIRE Investigators. Adenosine sestamibi SPECT post-infarction evaluation [INSPIRE] trial: a randomized, prospective multicenter trial evaluating the role of adenosine Tc-99m sestamibi SPECT for assessing risk and therapeutic outcomes in survivors of acute myocardial infarction. J Nucl Cardiol. 2004;11:458–69.
Farzaneh-Far A, Phillips HR, Shaw LK, Starr AZ, Fiuzat M, O’Connor CM, et al. Ischemia change in stable coronary artery disease is an independent predictor of death and myocardial infarction. JACC Cardiovasc Imaging. 2012;5:715–24.
Assante R, Acampa W, Zampella E, Arumugam P, Nappi C, Gaudieri V, et al. Coronary atherosclerotic burden vs. coronary vascular function in diabetic and nondiabetic patients with normal myocardial perfusion: a propensity score analysis. Eur J Nucl Med Mol Imaging. 2017;44:1129–35.
Acampa W, Gaemperli O, Gimelli A, Knaapen P, Schindler TH, Verberne HJ, et al. Document Reviewers. Role of risk stratification by SPECT, PET, and hybrid imaging in guiding management of stable patients with ischaemic heart disease: expert panel of the EANM cardiovascular committee and EACVI. Eur Heart J Cardiovasc Imaging. 2015;16:1289–98.
Zampella E, Acampa W, Assante R, Gaudieri V, Nappi C, Mannarino T, et al. Combined evaluation of regional coronary artery calcium and myocardial perfusion by 82Rb PET/CT in predicting lesion-related outcome. Eur J Nucl Med Mol Imaging. 2020;47:1698–704.
Maron DJ, Hochman JS, Reynolds HR, Bangalore S, O’Brien SM, Boden WE, et al. ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med. 2020;382:1395–407.
Funding
Open access funding provided by Università degli Studi di Napoli Federico II within the CRUI-CARE Agreement. Open access funding was provided by the University of Naples Federico II within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
R.A., M.P., A.C., and W.A. conceptualized the paper; E.Z., A.D.A., T.M., V.G., C.N., P.B., and Ma.P. evaluated and reported the imaging findings; R.A., M.P., A.C., and W.A. drafted the manuscript; and all the authors revised and commented on the paper and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Assante, R., Zampella, E., D’Antonio, A. et al. Impact on cardiovascular outcome of coronary revascularization-induced changes in ischemic perfusion defect and myocardial flow reserve. Eur J Nucl Med Mol Imaging 51, 1612–1621 (2024). https://doi.org/10.1007/s00259-023-06588-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06588-4