Abstract
Purpose
Dopamine transporter (DAT) imaging is used to support the diagnosis of neurodegenerative parkinsonian disorders. Specific medications have been reported to confound the interpretation of [123I]I-FP-CIT SPECT scans, but there is limited data. The aim of the current study is to identify potential medication effects on the interpretation of [123I]I-FP-CIT SPECT scans in routine practice.
Materials and methods
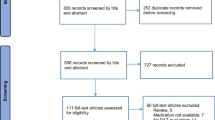
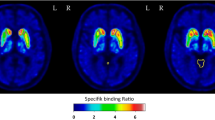
Consecutive patients undergoing a [123I]I-FP-CIT SPECT/CT scan on a 360° CZT camera between September 2019 and December 2022 were included. An exhaustive review of patient medications (antidepressants, antipsychotics, anti-epileptics, anti-parkinsonians, benzodiazepines, lithium, opioids, and stimulants) was performed. Two experienced nuclear physicians, blinded to the medication reports, interpreted the [123I]I-FP-CIT SPECT scans visually and a semi-quantitative analysis was performed using a local normal database.
Results
The study included 305 patients (71.0 ± 10.4, 135 women) and 145 (47.5%) visually interpreted normal scans. In normal scans, the striatum/occiput radioligand uptake ratio was decreased by noradrenergic and specific serotonergic antidepressants (NASSAs) (n = 15, z-score of − 0.93) and opioid medication (tramadol, n = 6, z-score of − 0.85) and was associated with a younger age in the multivariate analysis. In the overall population, the striatum/occiput ratio was influenced by NASSAs and associated with consensual visual analysis, age, sex, and anti-parkinsonian medications related to the status of the disease.
Conclusion
Our study confirms the potential impact of antidepressant (NASSA) and opioid (tramadol) medications on the semi-quantitative analysis of [123I]I-FP-CIT SPECT scans. However, when performing a visual analysis, only NASSAs significantly impacted the interpretation of [123I]I-FP-CIT SPECT scans.

Similar content being viewed by others
Data availability
Data that support the findings of this study may be requested from the corresponding author (AV).
Code availability
Not applicable.
References
Morbelli S, Esposito G, Arbizu J, Barthel H, Boellaard R, Bohnen NI, et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur J Nucl Med Mol Imaging. 2020;47:1885–912.
Verger A, Darcourt J, Habert M-O, Pallardy A, Santiago-Ribeiro M-J, Le Jeune F, et al. Guide de rédaction des protocoles d’examens d’imagerie de la neurotransmission pour l’exploration des mouvements anormaux. Médecine Nucl. 2021;45:98–111.
Chahid Y, Sheikh ZH, Mitropoulos M, Booij J. A systematic review of the potential effects of medications and drugs of abuse on dopamine transporter imaging using [123I]I-FP-CIT SPECT in routine practice. Eur J Nucl Med Mol Imaging. 2023;50(7):1974-1987. https://doi.org/10.1007/s00259-023-06171-x.
Booij J, Kemp P. Dopamine transporter imaging with [(123)I]FP-CIT SPECT: potential effects of drugs. Eur J Nucl Med Mol Imaging. 2008;35:424–38.
Aster H-C, Romanos M, Walitza S, Gerlach M, Mühlberger A, Rizzo A, et al. Responsivity of the striatal dopamine system to methylphenidate—a within-subject I-123-β-CIT-SPECT study in male children and adolescents with attention-deficit/hyperactivity disorder. Front Psychiatry. 2022;13: 804730.
Womack KB, Dubiel R, Callender L, Dunklin C, Dahdah M, Harris TS, et al. 123 I-Iofluopane single-photon emission computed tomography as an imaging biomarker of pre-synaptic dopaminergic system after moderate-to-severe traumatic brain injury. J Neurotrauma. 2020;37:2113–9.
Szobot CM, Shih MC, Schaefer T, Júnior N, Hoexter MQ, Fu YK, et al. Methylphenidate DAT binding in adolescents with attention-deficit/ hyperactivity disorder comorbid with substance use disorder - a single photon emission computed tomography with [Tc99m]TRODAT-1 study. Neuroimage. 2008;40:1195–201.
Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301:1148.
Borghammer P, Knudsen K, Danielsen E, Østergaard K. False-positive 123I-FP-CIT scintigraphy and suggested dopamine transporter upregulation due to chronic modafinil treatment. Clin Nucl Med. 2014;39:e87–8.
Learned-Coughlin SM, Bergström M, Savitcheva I, Ascher J, Schmith VD, Långstrom B. In vivo activity of bupropion at the human dopamine transporter as measured by positron emission tomography. Biol Psychiatry. 2003;54:800–5.
Árgyelán M, Szabó Z, Kanyó B, Tanács A, Kovács Z, Janka Z, et al. Dopamine transporter availability in medication free and in bupropion treated depression: A 99mTc-TRODAT-1 SPECT study. J Affect Disord. 2005;89:115–23.
Meyer JH, Goulding VS, Wilson AA, Hussey D, Christensen BK, Houle S. Bupropion occupancy of the dopamine transporter is low during clinical treatment. Psychopharmacology. 2002;163:102–5.
Hsiao M-C, Lin K-J, Liu C-Y, Beck SD. The interaction between dopamine transporter function, gender differences, and possible laterality in depression. Psychiatry Res Neuroimaging. 2013;211:72–7.
Honkanen EA, Kemppainen N, Noponen T, Seppänen M, Joutsa J, Kaasinen V. Bupropion causes misdiagnosis in brain dopamine transporter imaging for parkinsonism. Clin Neuropharmacol. 2019;42:181–3.
Booij J, de Jong J, de Bruin K, Knol R, de Win MML, van Eck-Smit BLF. Quantification of striatal dopamine transporters with 123I-FP-CIT SPECT is influenced by the selective serotonin reuptake inhibitor paroxetine: a double-blind, placebo-controlled, crossover study in healthy control subjects. J Nucl Med. 2007;48:359–66.
Shang Y, Gibbs MA, Marek GJ, Stiger T, Burstein AH, Marek K, et al. Displacement of serotonin and dopamine transporters by venlafaxine extended release capsule at steady state: a [123I]2β-carbomethoxy-3β-(4-iodophenyl)-tropane single photon emission computed tomography imaging study. J Clin Psychopharmacol. 2007;27:71–5.
Warwick JM, Carey PD, Cassimjee N, Lochner C, Hemmings S, Moolman-Smook H, et al. Dopamine transporter binding in social anxiety disorder: the effect of treatment with escitalopram. Metab Brain Dis. 2012;27:151–8.
Rominger A, Cumming P, Brendel M, Xiong G, Zach C, Karch S, et al. Altered serotonin and dopamine transporter availabilities in brain of depressed patients upon treatment with escitalopram: A [123I]β-CIT SPECT study. Eur Neuropsychopharmacol. 2015;25:873–81.
Wu C-K, Chin Chen K, See Chen P, Chiu N-T, Yeh TL, Lee IH, et al. No changes in striatal dopamine transporter in antidepressant-treated patients with major depression. Int Clin Psychopharmacol. 2013;28:141–4.
Krause D, Chrobok A, Karch S, Keeser D, Manz KM, Koch W, et al. Binding potential changes of SERT in patients with depression are associated with remission: a prospective [123I]β-CIT-SPECT study. Exp Clin Psychopharmacol. 2023;31:219–27.
Schmitt GJE, Dresel S, Frodl T, la Fougère C, Boerner R, Hahn K, et al. Dual-isotope SPECT imaging of striatal dopamine: a comparative study between never-treated and haloperidol-treated first-episode schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2012;262:183–91.
Mateos JJ, Lomeña F, Parellada E, Mireia F, Fernandez-Egea E, Pavia J, et al. Lower striatal dopamine transporter binding in neuroleptic-naive schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology. 2007;191:805–11.
Lavalaye J, Linszen DH, Booij J, Dingemans PMAJ, Reneman L, Habraken JBA, et al. Dopamine transporter density in young patients with schizophrenia assessed with [123]FP-CIT SPECT. Schizophr Res. 2001;47:59–67.
Chang WH, Chen KC, Lee IH, Chi MH, Chen PS, Yao WJ, et al. Unaltered dopamine transporter availability in drug-naive patients with schizophrenia after 6 months of antipsychotics treatment: a naturalistic study. J Clin Psychopharmacol. 2017;37:21–6.
Hou H, Yin S, Jia S, Hu S, Sun T, Chen Q, et al. Decreased striatal dopamine transporters in codeine-containing cough syrup abusers. Drug Alcohol Depend. 2011;118:148–51.
Piatkova Y, Payoux P, Boursier C, Bordonne M, Roch V, Marie P-Y, et al. Prospective paired comparison of 123I-FP-CIT SPECT images obtained with a 360°-CZT and a conventional camera. Clin Nucl Med. 2022;47:14–20.
Lanfranchi F, Arnaldi D, Miceli A, Mattioli P, D’Amico F, Raffa S, et al. Different z-score cut-offs for striatal binding ratio (SBR) of DaT SPECT are needed to support the diagnosis of Parkinson’s Disease (PD) and dementia with Lewy bodies (DLB). Eur J Nucl Med Mol Imaging. 2023;50:1090–102.
Kishi T, Iwata N. Meta-analysis of noradrenergic and specific serotonergic antidepressant use in schizophrenia. Int J Neuropsychopharmacol. 2014;17:343–54.
Croom KF, Perry CM, Plosker GL. Mirtazapine: a review of its use in major depression and other psychiatric disorders. CNS Drugs. 2009;23:427–52.
de Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacol. 1995;10:19–23.
Kent JM. SNaRIs, NaSSAs, and NaRIs: new agents for the treatment of depression. Lancet. 2000;355:911–8.
Fasipe O. Neuropharmacological classification of antidepressant agents based on their mechanisms of action. Arch Med Health Sci. 2018;6:81.
Millan MJ, Gobert A, Rivet J-M, Adhumeau-Auclair A, Cussac D, Newman-Tancredi A, et al. Mirtazapine enhances frontocortical dopaminergic and corticolimbic adrenergic, but not serotonergic, transmission by blockade of α 2 -adrenergic and serotonin 2C receptors: a comparison with citalopram: Monoamines and depression. Eur J Neurosci. 2000;12:1079–95.
Ziebell M, Holm-Hansen S, Thomsen G, Wagner A, Jensen P, Pinborg LH, et al. Serotonin transporters in dopamine transporter imaging: a head-to-head comparison of dopamine transporter SPECT radioligands 123 I-FP-CIT and 123 I-PE2I. J Nucl Med. 2010;51:1885–91.
Justesen TEH, Borghammer P, Aanerud J, Hovind P, Marner L. Sertraline treatment influences [18F]FE-PE2I PET imaging for Parkinsonism. EJNMMI Res. 2023;13:46.
Chen F, Lawrence AJ. The effects of antidepressant treatment on serotonergic and dopaminergic systems in Fawn-Hooded rats: a quantitative autoradiography study. Brain Res. 2003;976:22–9.
Thibaut F, Bonnet J-J, Vaugeois J-M, Costentin J. Pharmacological modifications of dopamine transmission do not influence the striatal in vivo binding of [3H]mazindol or [3H]cocaine in mice. Neurosci Lett. 1996;205:145–8.
Bergström KA, Jolkkonen J, Kuikka JT, Akerman KK, Viinamäki H, Airaksinen O, et al. Fentanyl decreases ?-CIT binding to the dopamine transporter. Synapse. 1998;29:413–5.
Beakley BD, Kaye AM, Kaye AD. Tramadol, pharmacology, side effects, and serotonin syndrome: a review. Pain Physician. 2015;18:395–400.
Hosseini-Sharifabad A, Rabbani M, Sharifzadeh M, Bagheri N. Acute and chronic tramadol administration impair spatial memory in rat. Res Pharm Sci. 2016;11:49–57.
Bameri B, Shaki F, Ahangar N, Ataee R, Samadi M, Mohammadi H. Evidence for the involvement of the dopaminergic system in seizure and oxidative damage induced by tramadol. Int J Toxicol. 2018;37:164–70.
Schillaci O, Pierantozzi M, Filippi L, Manni C, Brusa L, Danieli R, et al. The effect of levodopa therapy on dopamine transporter SPECT imaging with 123I-FP-CIT in patients with Parkinson’s disease. Eur J Nucl Med Mol Imaging. 2005;32:1452–6.
Innis RB, Marek KL, Sheff K, Zoghbi S, Castronuovo J, Feigin A, et al. Effect of treatment withL-dopa/carbidopa orL-selegiline on striatal dopamine transporter SPECT imaging with [123I]?-CIT. Mov Disord. 1999;14:436–42.
Nurmi E, Bergman J, Eskola O, Solin O, Hinkka SM, Sonninen P, et al. Reproducibility and effect of levodopa on dopamine transporter function measurements: a [18 F]CFT PET study. J Cereb Blood Flow Metab. 2000;20:1604–9.
Winogrodzka A. [123I]beta-CIT SPECT is a useful method for monitoring dopaminergic degeneration in early stage Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:294–8.
Fowler JS, Volkow ND, Logan J, Franceschi D, Wang G-J, MacGregor R, et al. Evidence that l-deprenyl treatment for one week does not inhibit mao a or the dopamine transporter in the human brain. Life Sci. 2001;68:2759–68.
Guttman M, Stewart D, Hussey D, Wilson A, Houle S, Kish S. Influence of L-dopa and pramipexole on striatal dopamine transporter in early PD. Neurology. 2001;56:1559–64.
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Parkinson study group. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351(24):2498–508. https://doi.org/10.1056/NEJMoa033447.
Rossi C, Genovesi D, Marzullo P, Giorgetti A, Filidei E, Corsini GU, et al. Striatal dopamine transporter modulation after rotigotine: results from a pilot single-photon emission computed tomography study in a group of early stage Parkinson disease patients. Clin Neuropharmacol. 2017;40:34–6.
Eusebio A, Azulay J-P, Ceccaldi M, Girard N, Mundler O, Guedj E. Voxel-based analysis of whole-brain effects of age and gender on dopamine transporter SPECT imaging in healthy subjects. Eur J Nucl Med Mol Imaging. 2012;39:1778–83.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the collection, analysis, and interpretation of the data (YP, MD, SH, LI, AV), to the writing of the manuscript (YP, AV) and to the revision of the manuscript (MD, AT, SF, LH, AV).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The institutional ethics committee (Comité d’Ethique du CHRU de Nancy) approved the evaluation of retrospective patient data and the trial was registered at ClinicalTrials.gov (NCT 05683665). This research complied with the principles of the Declaration of Helsinki. Informed consent was obtained from all individuals included in the study.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Piatkova, Y., Doyen, M., Heyer, S. et al. Effects of medication on dopamine transporter imaging using [123I]I-FP-CIT SPECT in routine practice. Eur J Nucl Med Mol Imaging 51, 1323–1332 (2024). https://doi.org/10.1007/s00259-023-06565-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06565-x