Abstract
Purpose
Despite the existence of various treatment options, the prognosis for patients with metastatic castration-resistant prostate cancer (mCRPC) remains unfavorable. One potential therapeutic approach is the use of [225Ac]Ac-PSMA-617, a targeted alpha therapy (TAT) that administers alpha-particle radiation specifically to prostate cancer cells expressing PSMA. In this study, we report the long-term survival outcomes of this novel therapy in a series of patients with mCRPC who have exhausted all standard treatment options.
Methods
The study enrolled patients with mCRPC who had shown resistance to standard lines of therapies, including next-generation anti-androgen therapies and taxane-based chemotherapies. These eligible patients received treatment with [225Ac]Ac-PSMA-617 at 100-150 kBq/kg doses administered every 8 weeks. The primary objective of the study was to assess overall survival (OS), while secondary objectives included evaluating radiological progression-free survival (rPFS), monitoring serum prostate-specific antigen (PSA) levels as a measure of biochemical response, and assessing adverse events using the CTCAE v5.0 grading system.
Results
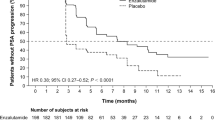
Among the 63 initially enrolled patients, a total of 56 patients who had completed at least two cycles of [225Ac]Ac-PSMA-617 were included in this study. The mean age was 67 years (range, 39-87) and patients received a total of 204 cycles of [225Ac]Ac-PSMA-617 TAT. 91% of patients exhibited any PSA decline, with 67.8% experiencing a decline of 50% or more. The median follow-up was of 22 months (range: 6-59 months). Imaging-based disease progression was observed in 68% of patients, and 66% of patients succumbed to the disease. The median OS was 15 months (95% CI: 10-19). In univariate analysis, factors such as lack of >50% PSA decline (P=0.031), Eastern Cooperative Oncology Group (ECOG) performance status of 2 or higher (P=0.048), and radiological progression (rPD) (P<0.001) were found to be predictors of poor OS. However, in multivariate analysis, only rPD emerged as an independent prognostic factor with a hazard ratio (HR) of 8.264 (95% CI: 1.429-16.497, P=0.004). The estimated median rPFS was 9 months (95% CI: 7-15). Moreover, patients who demonstrated any PSA decline had a median rPFS of 10 months compared to only 3 months in patients without any PSA decline (multivariate HR: 6.749; 95% CI: 1.949-23.370; P=0.002). Fatigue was one of the most common treatment-emergent adverse events, with grades 1/2 occurring in 70% of patients and grades 3 or higher in 3.5% of patients. This fatigue was transient and resolved before the next treatment cycle. Additionally, approximately one-third of patients experienced xerostomia (grades 1/2: 32.1%).
Conclusion
[225Ac]Ac-PSMA-617 targeted alpha therapy, was found to be well-tolerated with acceptable adverse events and effective in the treatment of patients with end-stage mCRPC.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Alam MR, Singh SB, Thapaliya S, et al. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as emerging theranostic agents in prostate cancer. Cureus 2022;14(9):e29369. https://doi.org/10.7759/cureus.29369.
Labriola MK, Atiq S, Hirshman N, Bitting RL. Management of men with metastatic castration-resistant prostate cancer following potent androgen receptor inhibition: a review of novel investigational therapies. Prostate Cancer Prostatic Dis. 2021;24:301–9.
Lawal IO, Bruchertseifer F, Vorster M, Morgenstern A, Sathekge MM. Prostate-specific membrane antigen-targeted endoradiotherapy in metastatic prostate cancer. Curr Opin Urol. 2020;30:98–105.
Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1091–1103.
Yadav MP, Ballal S, Sahoo RK, Dwivedi SN, Bal C. Radioligand Therapy With 177Lu-PSMA for Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Am J Roentgenol. 2019;213:275–85.
Kind F, Michalski K, Yousefzadeh-Nowshahr E, Meyer PT, Mix M, Ruf J. Bone marrow impairment during early [177Lu]PSMA-617 radioligand therapy: Haematotoxicity or tumour progression? EJNMMI Res. 2022;12:20.
Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225 Ac-PSMA-617 for PSMA-Targeted α-Radiation Therapy of Metastatic Castration-Resistant Prostate Cancer. J Nucl Med. 2016;57:1941–44.
Kratochwil C, Bruchertseifer F, Rathke H, et al. Targeted α-Therapy of Metastatic Castration-Resistant Prostate Cancer with 225 Ac-PSMA-617: Dosimetry Estimate and Empiric Dose Finding. J Nucl Med. 2017;58:1624–31.
Sathekge M, Bruchertseifer F, Knoesen O, et al. 225Ac-PSMA-617 in chemotherapy-naive patients with advanced prostate cancer: a pilot study. Eur J Nucl Med Mol Imaging. 2019;46:129–38.
Yadav MP, Ballal S, Sahoo RK, Tripathi M, Seth A, Bal C. Efficacy and safety of 225 Ac-PSMA-617 targeted alpha therapy in metastatic castration-resistant Prostate Cancer patients. Theranostics. 2020;10:9364–77.
Ma J, Li L, Liao T, Gong W, Zhang C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:796657.
Lee DY, Kim Y il. Effects of 225Ac-Labeled Prostate-Specific Membrane Antigen Radioligand Therapy in Metastatic Castration-Resistant Prostate Cancer: A Meta-Analysis. J Nucl Med. 2022;63:840–846.
Sathekge M, Bruchertseifer F, Vorster M, et al. mCRPC Patients Receiving 225 Ac-PSMA-617 Therapy in the Post–Androgen Deprivation Therapy Setting: Response to Treatment and Survival Analysis. J Nucl Med. 2022;63:1496–1502.
Sathekge M, Bruchertseifer F, Vorster M, et al. Predictors of Overall and Disease-Free Survival in Metastatic Castration-Resistant Prostate Cancer Patients Receiving 225Ac-PSMA-617 Radioligand Therapy. J Nucl Med. 2020;61:62–69.
Scher HI, Morris MJ, Stadler WM, et al. Trial Design and Objectives for Castration -Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34:1402–18.
Goldmacher G. (n.d.) Imaging Response Criteria Training for PCWG-Modified RECIST 1.1. Published by Articulate® Storyline. https://www.articulate.com. Accessed 24 July 2022.
McCaffery M &Pasero C. Pain: Clinical Manual. 2nd ed. St Louis, MO: Mosby; 1999.
Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:139 –44.
Common Terminology Criteria for Adverse Events (CTCAE) v5.0 Publish Date: November 27, 2017.
Ballal S, Yadav MP, Sahoo RK, Tripathi M, Dwivedi SN, Bal C. 225Ac-PSMA-617-targeted alpha therapy for the treatment of metastatic castration-resistant prostate cancer: A systematic review and meta-analysis. Prostate. 2021;81:580–91.
Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825–33.
Rathke, H., Kratochwil, C., Hohenberger, R. et al. Initial clinical experience performing sialendoscopy for salivary gland protection in patients undergoing 225Ac-PSMA-617 RLT. Eur J Nucl Med Mol Imaging. 2019;46:139–47.
Feuerecker B, Tauber R, Knorr K, et al. Activity and Adverse Events of Actinium-225-PSMA-617 in Advanced Metastatic Castration-resistant Prostate Cancer After Failure of Lutetium-177-PSMA. Eur Urol. 2021;79:343–50.
Author information
Authors and Affiliations
Contributions
The study's conception and design involved contributions from all authors. Sanjana Ballal, Madhav P Yadav, Swayamjeet Satapathy, and Shobhana Raju were responsible for patient enrollment, treatment, follow-up, material preparation, data collection, and analysis. The initial draft of the manuscript was written by Sanjana Ballal and Madhav Prasad Yadav. Chandrasekhar Bal, Madhavi Tripathi, and Nishikant A Damle processed, reported the images, and evaluated responses to treatment, Dr. Ranjit Kumar Sahoo was the Medical Oncologist and referred patients for treatment.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose
Disclosures
The authors have nothing to disclose.
Conflict of Interest
The authors have no conflict of interest
Ethical Clearance
Ethical clearance received Ref. No IEC-518/2018, RP-18/2018.
Informed Consent
Written informed consent was obtained from all patients to participate in the study and for the use of clinical information to analyze data.
Support
The Indian Council of Medical Research, under project No. 3/2/3/96/2019/NCD-III, provided funding support for a portion of the manpower involved in this work.
Disclaimer
This work has not been submitted elsewhere as a full article and is not under consideration by any other journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ballal, S., Yadav, M.P., Satapathy, S. et al. Long-term survival outcomes of salvage [225Ac]Ac-PSMA-617 targeted alpha therapy in patients with PSMA-expressing end-stage metastatic castration-resistant prostate cancer: a real-world study. Eur J Nucl Med Mol Imaging 50, 3777–3789 (2023). https://doi.org/10.1007/s00259-023-06340-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06340-y