Abstract
Purpose
Our aim was to assess the prognostic value of [68 Ga]Ga-FAPI-04 positron emission tomography (PET) uptake in PDAC and to evaluate the correlation between in vivo lesional radioactivity with pathological characteristics of pancreatic ductal adenocarcinoma (PDAC).
Methods
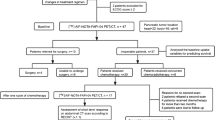
We retrospectively analyzed treatment‐naïve PDAC patients who underwent preoperative [68 Ga]Ga-FAPI-04 PET/CT followed by pancreatectomy. The tracer uptake was determined as maximum tumor standardized uptake value (SUVmax), FAPI-avid tumor volume (FTV), total lesion FAP expression (TLF) as well total pancreatic uptake (TSUVmax), total FAPI-avid pancreatic volume (FPV), and total pancreatic FAP expression (TPF). Spearman’s correlation analysis was performed to evaluate the association between [68 Ga]Ga-FAPI-04 PET/CT imaging and ex vivo immunohistological FAP expression and pathological characteristics of surgical specimens (differentiation, size, vascularity, perineural invasion, and lymph node metastases). Kaplan–Meier and hazard ratio (HR, log-rank) methods were used to evaluate the prognostic value of [68 Ga]Ga-FAPI-04 PET/CT and clinicopathological factors.
Results
Thirty-seven surgical PDAC patients were included. The ex vivo expression of FAP was significantly associated with the tumor SUVmax and TLF. FAP expression was more abundant in poorly differentiated PDAC than in well- to moderately differentiated neoplasms. Tumor SUVmax or TLF and pancreatic TSUVmax or TPF were significantly correlated with tumor size, differentiation, and perineural invasion, respectively. SUVmax had a significant independent prognostic value for recurrence-free survival (HR = 2.46, P < 0.05), while [68 Ga]Ga-FAPI-04 TPF predicted overall survival (HR = 12.82, P < 0.05).
Conclusion
The in vivo [68 Ga]Ga-FAPI-04 uptake in localized PDAC showed a significant correlation with ex vivo FAP expression and aggressive pathological characteristics. [68 Ga]Ga-FAPI-04 PET/CT also presented a potential for postoperative prognostication of PDAC. Elevated fibroblast activity induced by obstructive pancreatitis might be associated with the patient's survival.






Similar content being viewed by others
Data availability
The datasets generated or analyzed during this study are available from the corresponding author on reasonable request.
References
Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–20. https://doi.org/10.1016/S0140-6736(20)30974-0.
Groot VP, Rezaee N, Wu W, Cameron JL, Fishman EK, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–45. https://doi.org/10.1097/SLA.0000000000002234.
Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17(8):487–505. https://doi.org/10.1038/s41575-020-0300-1.
Nicolle R, Blum Y, Marisa L, Loncle C, Gayet O, et al. Pancreatic adenocarcinoma therapeutic targets revealed by tumor-stroma cross-talk analyses in patient-derived xenografts. Cell Rep. 2017;21(9):2458–70. https://doi.org/10.1016/j.celrep.2017.11.003.
Madsen CD. Pancreatic cancer is suppressed by fibroblast-derived collagen I. Cancer Cell. 2021;39(4):451–3. https://doi.org/10.1016/j.ccell.2021.02.017.
Niedermeyer J, Kriz M, Hilberg F, Garin-Chesa P, Bamberger U, et al. Targeted disruption of mouse fibroblast activation protein. Mol Cell Biol. 2000;20(3):1089–94. https://doi.org/10.1128/MCB.20.3.1089-1094.2000.
Luo Y, Pan Q, Yang H, Peng L, Zhang W, et al. Fibroblast activation protein-targeted PET/CT with 68Ga-FAPI for imaging IgG4-related disease: comparison to 18F-FDG PET/CT. J Nucl Med. 2021;62(2):266–71. https://doi.org/10.2967/jnumed.120.244723.
Feng Q, Li C, Zhang S, Tan CL, Mai G, et al. Recurrence and survival after surgery for pancreatic cancer with or without acute pancreatitis. World J Gastroenterol. 2019;25(39):6006–15. https://doi.org/10.3748/wjg.v25.i39.6006.
Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw. 2019;17(5.5):603–5. https://doi.org/10.6004/jnccn.2019.5007.
Shi X, Xing H, Yang X, Li F, Yao S, Zhang H, et al. Fibroblast imaging of hepatic carcinoma with (68)Ga-FAPI-04 PET/CT: a pilot study in patients with suspected hepatic nodules. Eur J Nucl Med Mol Imaging. 2021;48(1):196–203. https://doi.org/10.1007/s00259-020-04882-z.
Mokoala KMG, Lawal IO, Lengana T, Popoola GO, Boshomane TMG, et al. The association of tumor burden by 18F-FDG PET/CT and survival in vulvar carcinoma. Clin Nucl Med. 2021;46(5):375–81. https://doi.org/10.1097/RLU.0000000000003549.
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, WHO Classification of Tumours Editorial Board, et al. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2020;76(2):182–8. https://doi.org/10.1111/his.13975.
Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144(6):1210–9. https://doi.org/10.1053/j.gastro.2012.11.037.
Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, et al. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248(1):51–65. https://doi.org/10.1002/path.5224.
Menezes S, Okail MH, Jalil SMA, Kocher HM, Cameron AJM. Cancer-associated fibroblasts in pancreatic cancer: new subtypes, new markers, new targets. J Pathol. 2022;257(4):526–44. https://doi.org/10.1002/path.5926.
Fitzgerald AA, Weiner LM. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020;39(3):783–803. https://doi.org/10.1007/s10555-020-09909-3.
Henry LR, Lee HO, Lee JS, Klein-Szanto A, Watts P, Ross EA, et al. Clinical implications of fibroblast activation protein in patients with colon cancer. Clin Cancer Res. 2007;13(6):1736–41. https://doi.org/10.1158/1078-0432.CCR-06-1746.
Nurmik M, Ullmann P, Rodriguez F, Haan S, Letellier E. In search of definitions: cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146(4):895–905. https://doi.org/10.1002/ijc.32193.
Cohen SJ, Alpaugh RK, Palazzo I, Meropol NJ, Rogatko A, Xu Z, et al. Fibroblast activation protein and its relationship to clinical outcome in pancreatic adenocarcinoma. Pancreas. 2008;37(2):154–8. https://doi.org/10.1097/MPA.0b013e31816618ce.
Kawase T, Yasui Y, Nishina S, Hara Y, Yanatori I, Tomiyama Y, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015;15:109. https://doi.org/10.1186/s12876-015-0340-0.
Shi M, Yu DH, Chen Y, Zhao CY, Zhang J, Liu QH, et al. Expression of fibroblast activation protein in human pancreatic adenocarcinoma and its clinicopathological significance. World J Gastroenterol. 2012;18(8):840–6. https://doi.org/10.3748/wjg.v18.i8.840.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60(6):801–5. https://doi.org/10.2967/jnumed.119.227967.
Herreros-Villanueva M, Gironella M, Castells A, Bujanda L. Molecular markers in pancreatic cancer diagnosis. Clin Chim Acta. 2013;418:22–9. https://doi.org/10.1016/j.cca.2012.12.025.
Chikamoto A, Inoue R, Komohara Y, Sakamaki K, Hashimoto D, Shiraishi S, et al. Preoperative high maximum standardized uptake value in association with glucose transporter 1 predicts poor prognosis in pancreatic cancer. Ann Surg Oncol. 2017;24(7):2040–6. https://doi.org/10.1245/s10434-017-5799-1.
Dunet V, Halkic N, Sempoux C, Demartines N, Montemurro M, Prior JO, et al. Prediction of tumour grade and survival outcome using pre-treatment PET- and MRI-derived imaging features in patients with resectable pancreatic ductal adenocarcinoma. Eur Radiol. 2021;31(2):992–1001. https://doi.org/10.1007/s00330-020-07191-z.
Smith RA, Bosonnet L, Ghaneh P, Raraty M, Sutton R, Campbell F, et al. Preoperative CA19–9 levels and lymph node ratio are independent predictors of survival in patients with resected pancreatic ductal adenocarcinoma. Dig Surg. 2008;25(3):226–32. https://doi.org/10.1159/000140961.
Lee JW, Kang CM, Choi HJ, Lee WJ, Song SY, et al. Prognostic value of metabolic tumor volume and total lesion glycolysis on preoperative 18F-FDG PET/CT in patients with pancreatic cancer. J Nucl Med. 2014;55(6):898–904. https://doi.org/10.2967/jnumed.113.131847.
Yamamoto T, Sugiura T, Mizuno T, Okamura Y, Aramaki T, et al. Preoperative FDG-PET predicts early recurrence and a poor prognosis after resection of pancreatic adenocarcinoma. Ann Surg Oncol. 2015;22(2):677–84. https://doi.org/10.1245/s10434-014-4046-2.
Funding
This work was sponsored in part by the National Natural Science Foundation of China (Grant No. 82071967), the National Key Research and Development Program of China (Grant No. 2020YFC2002702), CAMS Initiative for Innovative Medicine (No. CAMS-2018-I2M-3–001), CAMS initiative for innovative medicine (2016ZX310174-4), Tsinghua University-Peking Union Medical College Hospital Initiative Scientific Research Program (Grant No. 52300300519), and Capital’s Funds for Health Improvement and Research (CFH-2018–2-4014).
Author information
Authors and Affiliations
Contributions
All authors were involved in the study conception and design. Jie Ding, Zhixin Hao, Hua Huang, Qiaofei Liu Wenjing Liu, and Chao Ren were involved in acquisition of data. Jie Ding and Jiangdong Qiu were involved in analysis and interpretation of the data. Jie Ding, Xiang Li, and Jiangdong Qiu were involved in drafting of the manuscript. All authors were involved with critical revisions of the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All procedures performed in studies involving human participants were in accordance with the Institutional Ethics Committee of the Peking Union Medical College Hospital (Beijing, China, IRB protocol #ZS1810).
Consent to participate
Informed consent was obtained from all individual participants included in the study for publication.
Consent for publication
The authors affirm that human research participants provided informed consent for publication of the images in this article.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ding J and Li X shared the authorship.
Jie Ding is the first author.
This article is part of the Topical Collection on Oncology - Digestive tract
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ding, J., Qiu, J., Hao, Z. et al. Prognostic value of preoperative [68 Ga]Ga-FAPI-04 PET/CT in patients with resectable pancreatic ductal adenocarcinoma in correlation with immunohistological characteristics. Eur J Nucl Med Mol Imaging 50, 1780–1791 (2023). https://doi.org/10.1007/s00259-022-06100-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-06100-4