Abstract
Purpose
Treatment strategies of lymphoid malignancies have been revolutionized by immunotherapy. Because of the inherent property of Hodgkin lymphoma and some subtypes of non-Hodgkin lymphoma as a highly FDG-avid tumor, functional 18F-FDG PET/CT imaging is already embedded in their routine care. Nevertheless, the question is whether it is still valuable in the context of these tumors being treated with immunotherapy. Herein, we will review the value of 18F-FDG PET/CT imaging lymphoid tumors treated with immunotherapy regimens.
Methods
A comprehensive literature search of the PubMed database was conducted on the value of the 18F-FDG PET/CT for immunotherapy response monitoring of patients with malignant lymphoma. The articles were considered eligible if they met all of the following inclusion criteria: (a) clinical studies on patients with different types of malignant lymphoma, (b) treatment with anti-CD20 antibodies, immune checkpoint inhibitors or immune cell therapies, (c) and incorporated PET/CT with 18F-FDG as the PET tracer.
Results
From the initial 1488 papers identified, 91 were ultimately included in our study. In anti-CD20 therapy, the highest pooled hazard ratios (HRs) of baseline, early, and late response monitoring parameters for progression-free survival (PFS) belong to metabolic tumor volume (MTV) (3.19 (95%CI: 2.36–4.30)), maximum standardized uptake value (SUVmax) (3.25 (95%CI: 2.08–5.08)), and Deauville score (DS) (3.73 (95%CI: 2.50–5.56)), respectively. These measurements for overall survival (OS) were MTV (4.39 (95%CI: 2.71–7.08)), DS (3.23 (95%CI: 1.87–5.58)), and DS (3.64 (95%CI: 1.40–9.43)), respectively. Early and late 18F-FDG PET/CT response assessment in immune checkpoint inhibitors (ICI) and immune cell therapy might be an effective tool for prediction of clinical outcome.
Conclusion
For anti-CD20 therapy of lymphoma, the MTV as a baseline 18F-FDG PET/CT-derived parameter has the highest HRs for PFS and OS. The DS as visual criteria in early and late response assessment has higher HRs for PFS and OS compared to the international harmonization project (IHP) visual criteria in anti-CD20 therapy. Early changes in 18F-FDG PET parameters may be predictive of response to ICIs and cell therapy in lymphoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Treatment strategies of the lymphoid malignancies were revolutionized by immunotherapy in two major advances over the last 3 decades. In 1997, the introduction of anti-CD20 monoclonal antibodies (mAbs) such as rituximab which targeted B-cells exclusively to evoke a direct anti-tumor cytotoxic effect revolutionalized the treatment paradigm for lymphoma. Then, in 2017, the introduction of immune checkpoint inhibitors (ICI) such as anti-PD1 and anti-CTLA4 monoclonal antibodies which stimulates the immune system via T cells appears to be the next promising step in lymphoma management [1,2,3]. Although immunotherapy now is common terminology for the class of drugs that stimulate the immune system to indirectly target cancer cells, conventionally, immunotherapy refers to any therapy that manipulates the immune system such as both anti-CD20 mAbs and new targeted immunotherapy, ICIs, and cell therapy.
Anti-CD20 monoclonal antibodies have FDA approval for treatment of non-Hodgkin lymphoma (NHL) for multiple indications as monotherapy or in combination with other lymphoma-directed therapeutics [4, 5], and have a place in treatment paradigms for many B cell lymphomas [6]. Among the immune checkpoint inhibitors, pembrolizumab and nivolumab have regulatory approval for the treatment management of the relapsed or refractory transplant ineligible or post-transplant relapsed classical Hodgkin lymphoma (HL) and relapsed or refractory primary mediastinal B cell lymphoma (PMBL) [7, 8]. CD19-directed chimeric antigen receptor (CAR) T cell therapy is approved for the treatment of certain types of large B-cell lymphoma relapsed or refractory to at least two other treatment regimens [9].
Because of the inherent property of HL and most subtypes of NHL as highly 18F-FDG avid tumors, functional 18F-FDG PET/CT imaging is a standard response assessment tool for these diseases and has a decisive role in noninvasive response monitoring of therapies, including immunotherapeutics [10, 11].
In the abovementioned framework, multiple different visual 18F-FDG PET criteria were introduced to more consistently evaluate 18F-FDG PET scans [10, 12]. Despite the available 18F-FDG PET criteria, following the introduction of ICIs some modified response assessment criteria were proposed. These criteria were developed based on the observation that some immunomodulatory drugs can alter tumoral glucose metabolism, changing the assumed association between the 18F-FDG uptake and treatment efficacy observed under conventional chemotherapy [2].
As evident from the literature, existing original articles have some differences in the population and methodologies. Specifically, the malignant lymphoma analysed (either HL or NHL), immunotherapy regimens and line of therapy (anti-CD20 mAbs, ICI versus immune cell therapy), and time points and time intervals of performing 18F-FDG PET scan (baseline, early, and late 18F-FDG PET imaging), all vary considerably.
Various studies have dealt with the issue of the value of metabolic imaging by 18F-FDG PET scan for immunotherapy management in malignant lymphoma patients, but the evidence for lymphoma treated with ICIs is lacking due to the current standard being that of a quantitative or semi-quantitative assessment, primarily using the Lugano or LYRIC criteria [13, 14]. Here, we will review the role of 18F-FDG PET imaging in response assessment or response prediction in the lymphoid tumors treated with immunotherapy regimens using the perspective of the mentioned studies.
Methods
Literature search
A comprehensive literature search of the PubMed database was conducted to retrieve relevant published articles concerning the value of the 18F-FDG PET/CT for response monitoring of patients with malignant lymphoma to immunotherapy including anti-CD20 therapy, ICI therapy, and cell therapy. The search was based on the various combinations of the Boolean operators and the following keywords “lymphoma,” “Hodgkin disease,” “Hodgkin lymphoma,” “non-Hodgkin lymphoma,” “18F-FDG PET/CT,” “positron emission tomography,” “immunotherapy,” “immune checkpoint inhibitors,” “anti-CD20 therapy,” “CAR T cell therapy,” “Rituximab,” “Nivolumab,” “Ipilimumab,” “Pembrolizumab”…. No date or language restriction was applied and the search was updated until March 2021. The reference list of the eligible articles was manually screened to identify any pertinent study.
Eligibility criteria
The relevant original articles were considered eligible if they met all of the following inclusion criteria: (a) clinical studies on patients with different types of malignant lymphoma including Hodgkin disease and non-Hodgkin lymphoma; (b) treatment with anti CD-20 mAbs, ICIs, or cell therapy; and (c) incorporation of PET/CT with the 18F-FDG as the PET tracer. The exclusion criteria were as follows: (a) investigations on animals, (b) radioimmunotherapy as treatment, (c) PET/CT imaging with PET tracers other than 18F-FDG, (d) articles without sufficient data regarding performed 18F-FDG PET/CT, (e) duplicated articles, (f) CNS lymphoma due to physiologic high 18F-FDG uptake in the central nervous system, and (g) HIV-related lymphoma.
Data extraction and quality assessment
The required study characteristics were extracted by reviewing the whole text of the eligible articles. The gathered data were arranged in three main parts: basic study characteristics including the name of the first author and publication date; demographic information including the number of participants, lymphoma subtype, and immunotherapy regimen; and the technical aspects including 18F-FDG PET imaging method and findings, response assessment criteria or technique, outcomes, and hazard ratios (HR). In the cases that HR was not directly reported using depicted Kaplan–Meier curve, Graph Digitizer version 2.24, Richard Steven’s excel workbook, the HR and its 95% CI were estimated. The quality of all eligible articles was evaluated by employing the established critical appraisal tool obtained from the Oxford Center for Evidence-Based Medicine [15]. This tool was designed to evaluate the quality of the prognostic studies by taking into account several factors consisting of patient registration time, follow-up duration, outcome criteria, and adjustment for important prognostic factors. All quality assessments have been tabulated in the supplemental table 1 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106].
For metabolic baseline parameters, we considered tumor burden indices, including metabolic tumor volume (MTV), and total lesion glycolysis (TLG), and tumor metabolism indices, including maximum of standardized uptake value (SUVmax). For the response assessment we considered visual methods, including Deauville score (DS) and ΔSUVmax as a semi-quantitative method [21, 22, 48, 67, 77, 80, 89, 104].
Statistical analysis
The statistical analyses for pooling hazard ratios were carried out using Comprehensive meta-analysis software (CMA version 2). The random effects model was used to pool effect sizes across included studies. Heterogeneity was evaluated using Cochrane Q value (p-values less than 0.05 were considered statistically significant) and I2 index. Publication bias was evaluated graphically using funnel plots. Because of discrepancies in methodological aspects of the included articles, the evaluation of publication bias was not possible for all papers, and only was performed for studies with similar reported indices (for more details see supplemental figures file).
Results
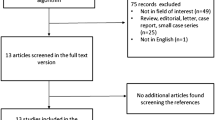
The performed search approach is presented as a PRISMA flowchart [107] in Fig. 1. From the initial 1488 identified papers, 91 were ultimately included in our study. As we expected, the eligible included studies showed discrepancies in methodological aspects and the main remarkable one was the applied treatment strategy. In the first step, the enrolled studies were classified into studies on anti-CD20 monoclonal antibodies (for example, rituximab), studies on ICIs (for example, nivolumab), and studies on cellular therapies (for example, CAR T-cell therapy or dendritic cell therapy). The former part involved 77 publications, whereas the two latter ones included a total of 14 investigations. Basic study characteristics of each part are separately listed in three tables (Supplemental Table 234) [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106].
Anti-CD20 immunotherapy
Baseline metabolic parameters
Among 77 included articles in the anti-CD20 immunotherapy group, 29 studies investigated the value of the metabolic baseline parameters including tumor burden indices, metabolic tumor volume (MTV), and total lesion glycolysis (TLG), as well as tumor metabolism indices, maximum of standardized uptake value (SUVmax). Significant association with progression-free survival (PFS) and overall survival (OS) was reported for both MTV and TLG in 5 articles [25, 27, 32, 104, 105]. Several articles found significant correlation between MTV [31, 52, 61, 67, 74, 76, 77, 83, 84, 92, 93, 101, 103, 106], TLG [42, 53, 61, 67, 74, 101], and SUVmax (23,35, 9,43) [43, 84, 104] with PFS. Furthermore, a significant correlation between MTV [16, 31, 76, 83, 84, 92, 93, 103], TLG [53], and SUVmax [84] with OS was reported as well. Furthermore, high levels of MTV and TLG were related to the probability of central nervous system relapse [82]. In addition, some studies indicated the combination of the baseline PET-derived metabolic parameters with patients clinical features and laboratory data [62], as well as other baseline parameters [31, 78] or interim [67, 101] or EOT response [74], can better stratify lymphoma patients outcome. Notably, a significant relation with one or two of these parameters was not essentially found in some of the mentioned investigations. One paper reported that these baseline parameters do not provide prognostic information beyond that can already be obtained by the International Prognosis Index (IPI) [16].
According to the forest plot of the effect of baseline metabolic parameters on clinical outcome of patients treated with anti-CD20 immunotherapy, depicted in Figs. 2 and 3, the included studies reported quite heterogeneous hazard ratios from 1.1 to 16.73. On the basis of the meta-analysis calculations for PFS, one finds that the values of the pooled HRs of baseline MTV, TLG, and SUVmax were equal to 3.19 (95%CI: 2.36–4.30; P = 0.000), 2.54 (95%CI: 1.57–4.12; P = 0.000), and 1.18 (95%CI: 0.79–1.75; P = 0.404), respectively, while, for OS, the pooled HRs were 4.39 (95%CI: 2.71–7.08; P = 0.000), 2.68 (95%CI: 1.93–3.72; P = 0.000), and 1.65 (95%CI: 1.02–2.69; P = 0.041) for MTV, TLG, and SUVmax, respectively.
Bone marrow involvement based on hypermetabolic lesions in the bone marrow on baseline 18F-FDG PET/CT imaging is another factor which is predictive of clinical outcomes and was previously assessed in some studies [29, 30, 41, 50, 75, 81]. A poorer clinical outcome in patients with bone marrow 18F-FDG avid loci was reported by three investigations [29, 41, 50]. Likely, a poor outcome was associated with more than 2 sites of extra nodal involvement in baseline 18F-FDG PET/CT imaging [40, 41]. Another study revealed histological transformation into aggressive lymphoma types in follicular lymphoma did not have any relation with SUVmax or SUV range in baseline 18F-FDG PET/CT imaging [69].
According to the funnel plots, publication bias is probable for the pooled HR of MTV, TLG, and SUVmax for PFS as well as MTV for OS (supplemental figures).
Interim response assessment
A total of 38 articles investigated response assessment via early 18F-FDG PET/CT imaging in the anti-CD20 immunotherapy, and of these, 19 studies were included in the meta-analysis (Supplemental Table 2) [16, 18, 21,22,23, 25,26,27, 29,30,31,32,33,34,35, 38,39,40,41,42,43,44,45,46, 48,49,50,51,52,53,54,55,56,57, 59,60,61,62,63,64,65,66,67,68,69, 71,72,73,74,75,76,77,78, 80,81,82,83,84,85,86,87,88,89,90,91,92, 96,97,98,99,100,101,102,103,104,105,106]. The meta-analysis was carried out on the DS, IHP, and ΔSUVmax for PFS and OS, and the obtained results are depicted as forest plots in Figs. 4 and 5. The pooled HR of the ΔSUVmax was the highest value for PFS of 3.25 (95%CI: 2.08–5.08; P = 0.000). However, for OS, the DS had the highest HR of 3.23 (95%CI: 1.87–5.58; P = 0.000). The pooled HRs of the IHP for PFS and OS were the lowest values, being 2.23 (95%CI: 1.04–4.78; P = 0.039) and 1.83 (95%CI: 0.64–5.19; P = 0.254), respectively.
Several studies have reported that interim 18F-FDG PET/CT could have a key role in survival prediction and early response assessment of patients with lymphoma treated with anti-CD20 therapy [21, 38, 48, 49, 55, 56, 64, 80, 99, 100, 106]. In contrast, several studies reported a poor positive predictive value in early response assessment [45, 46, 54, 60, 66, 72, 97, 104], whereby the interpretation of the 18F-FDG PET/CT scan is of special importance. Indeed, the results obtained through visual analysis using IHP or DS criteria have shown poor positive predictive value for recurrence compared to the semi-quantitative analysis based on SUVmax values [22, 23, 38, 45, 48]. Moreover, the combination of clinical variables and either interim-derived quantitative parameters or interim DS has been shown to improve the value of outcome prediction [56, 73, 85, 99]. In confirmation of this finding, the subgroup meta-analysis performed for interim PET scan showed the highest HR belonged to ΔSUVmax for prediction of both PFS and OS. Several studies reported a high negative predictive value for early time point 18F-FDG PET/CT imaging [23, 35, 68, 89]. Subsequently, some investigations reported conventional treatment intensification based on interim 18F-FDG PET/CT findings might had not clinical benefit and could not improve the final outcome and must be restricted to clinical trials [38, 86].
Early 18F-FDG PET/MRI using quantitative 18F-FDG PET and diffusion-weighted magnetic resonance imaging parameters has been shown to identify anti-CD20 therapy-induced changes as early as 48–72 h after treatment initiation [63, 64].
The presence of publication bias for the pooled HR of interim response assessment using DS for OS is probable (supplemental figures).
End of treatment (EOT) response assessment
Meta-analysis was performed using 16 articles on anti-CD20 therapy. The reported HRs, for PFS, are widely ranged from 1.27 to 14.54 and 1 to 11.58 for DS and IHP, respectively. For PFS, the pooled HR of the EOT response assessment using DS and IHP was 3.73 (95%CI: 2.50–5.56; P = 0.000) and 2.60 (95%CI: 1.82–3.71; P = 0.000), respectively. For OS, this range was from 0.89 to 12.21 for DS and from 0.61 to 5.26 for IHP criteria. The pooled HR was 3.64 (95%CI: 1.40–9.43; P = 0.008) and 2.01 (95%CI: 0.67–6.00; P = 0.208), respectively. The corresponding forest plots were illustrated in Figs. 6 and 7.
For EOT 18F-FDG PET/CT as well, the reports on potential prognostic effect have been mixed. Several studies reported the EOT 18F-FDG PET/CT using different evaluation systems could have a promising role in outcome prediction [18, 23, 44, 54, 57, 60, 65, 74, 87, 88, 90, 91, 98, 104]. Aside from visual criteria, some newer quantitative parameters such as the ratio between the SUVmax of a hypermetabolic lymphoma lesion and liver SUVmax on the EOT 18F-FDG PET/CT have a similar prognostic power as DS in predicting of the patient clinical outcome [18, 89]. On the other hand, some investigations reported a low positive predictive value for EOT imaging, which could limit the role of 18F-FDG PET/CT in end of treatment response evaluation [26, 45, 46, 59, 65]. Similar to the interim imaging, a high NPV was reported on the EOT scan [46, 49, 65, 66, 68, 85, 104].
Addition of rituximab to the conventional chemotherapy resulted in reduced positive predictive value of the interim and end of treatment 18F-FDG PET/CT-based response assessment [45, 46]. This may in part be explained by the inflammatory reactions as a consequence of recruitment of immune cells to the tumor following rituximab treatment. Consequently, glucose consumption of the migrated immune cells might lead to a local hypermetabolism and false-positive PET results [46].
Immune checkpoint inhibitors (ICI)
Baseline metabolic parameters
Unlike the large number of studies on anti-CD20 therapy, there are fewer studies on the value of 18F-FDG PET/CT on response assessment of ICIs in lymphoma. These results in a limitation of small sample sizes and short follow-up duration since ICI is a more recently adopted therapy. Among the nine included articles under this category, one reported that baseline MTV had no statistically significant correlation with achieving complete metabolic response after pembrolizumab therapy [17].
Response assessment
In the category of ICIs, early 18F-FDG PET/CT scans appear to be more important than end of treatment response assessment. Some studies stated that patients achieved objective response after 3 months of ICI initiation and early 18F-FDG PET/CT evaluation can detect responders at this time point [19, 36]. Various methods have been employed to assess early imaging including quantitative (MTV) or qualitative (DS) approaches; however, early 18F-FDG PET/CT findings were associated with the best overall response [19, 24, 36, 94] or patient survival [24, 28, 58, 70]. Reduction of MTV [24, 36, 94], ΔSUVmax [24, 36], decrease in TLG (92) [24], and visual analysis by DS [24, 70] was associated with the best overall response (Supplemental Table 3) [17, 19, 24, 28, 36, 58, 70, 94]. Although some of studies evaluated Lugano and LYRIC criteria, they were heterogeneous in methodology and reported results; so we could not enrolled them to meta-analysis [24, 28, 36, 70].
Immune-related adverse events (irAEs)
The potential irAEs related to ICI treatment include fever, headache, arthralgia, cytopenia, respiratory system involvement, gastrointestinal tract involvement, and skin rash [17, 19, 24, 58]. In one study, it was reported that the rate of irAE was higher in ICI responding patients in comparison to the non-responders; however, this finding was not significant [24]. There were no reports on irAEs leading to death in the articles reviewed [17, 24, 58].
Cellular therapy
Baseline metabolic parameters
In cell therapy, among the 8 related studies, only one indicated MTV had a significant association with PFS and OS [93]. On the other hand, one article stated MTV and TLG were not associated with response to treatment and OS [95].
Response assessment
Early 18F-FDG PET/CT imaging at ~ 1 month after therapy initiation has been reported to be useful for early response assessment following cellular therapy and may have a role in guiding treatment pathway [37, 79]. For example, MTV may be an effective tool for early response assessment [79] and early suppression of glucose consumption in lymphoid organs may be associated with poor outcome [37]. Also, 18F-FDG PET may have a role in monitoring the immune effects after allogeneic cell transplantation [47] (Supplemental Table 4) [20, 37, 47, 79, 93, 95].
Immune-related adverse events (irAEs)
The most common irAEs of CAR-T cell therapy is cytokine release syndrome (CRS), and a concurrent local immune activation within a tumoral lesion that can cause a severe local inflammation which can lead to pseudo-progression. It has been reported that the baseline tumor burden was linked to CRS and pseudo-progression in treatment course [37]. It should be noted that there is a controversy in this issue and another study did not witness any relation between baseline FDG metabolic parameters and CRS [79]. Another study reported that the presence of pseudo-progression did not alter early response evaluation [58]. Moreover, another study indicated that CRS did not confound 18F-FDG PET/CT interpretation [47].
Discussion
The high sensitivity of the 18F-FDG PET in detecting nodal and extranodal involvement has established its role in primary staging as a standard of care for all 18F-FDG-avid lymphomas [11]. Calculating quantitative baseline tumor burden and tumor metabolism indices following immunotherapy are common parameters used to predict prognosis in baseline 18F-FDG PET scans [25, 27, 32, 104, 105]. However, some studies have not found a definite correlation between one or more of these parameters with PFS or OS [37, 95]. This can, in part, be explained by heterogeneous patient characteristics, including a wide age range, different clinical stage and disease subtypes, and different software and the different ways used for definition of the marginal threshold of hypermetabolic foci [27]. Moreover, these discrepancies are obvious through the heterogeneity of reported HRs in Figs. 2 and 3. Based on the meta-analysis performed in the present work, the highest pooled HR between baseline parameters belongs to MTV with HR of 4.39 (95%CI: 2.71–7.08; P = 0.000] and the lowest one belongs to SUVmax with an HR of 1.18 (95%CI: 0.79–1.75; P = 0.404).
Many reports suggest response assessment via semi-quantitative/quantitative methods in lymphoma patients provides better outcome discriminators than the visual criteria regardless of the considered background reference tissue [21, 22, 38, 48]. Moreover, it has been reported that inter-observer reproducibility was higher using semi quantitative methods than the visual approaches [48]. Our findings showed the pooled HR of the early response assessment following anti-CD20 treatment using ΔSUVmax for PFS is 3.25 (95%CI: 2.08–5.08; P = 0.000), which is higher than the DS and IHP criteria’s pooled HRs. Pooled HR for an early evaluation concerning OS did not support this point and the pooled HR of the ΔSUVmax is lower than the DS corresponding value, 2.87 (95%CI: 1.92–4.28; P = 0.000] versus 3.23 (95%CI: 1.87–5.58, P = 0.000). On the other hand, according to some studies, it seems that visual assessment by DS is a better prognosticator than the IHP criteria [45]. Our findings in early response assessment pooled HRs also support this point. Pooled HR of the early response assessment for PFS and OS are respectively 2.56 (95%CI: 1.79–3.66; P = 0.000] and 3.23 (95%CI: 1.87–5.58; P = 0.000) for DS, which are higher than the IHP criteria’s pooled HRs. These measurements for DS and IHP were relatively similar for EOT 18F-FDG PET/CT. This difference can in part be explained by the more conservative nature of the IHP criteria, which uses a lower visual reference (surrounding background or mediastinal blood pool) compared to the DS, which uses liver parenchymal uptake [18]. It should be mentioned that the SUV of the reference organs may be affected by the hypermetabolic tumor burden, and this point should be considered when interpreting serial 18F-FDG PET/CT scans [51].
In immunotherapy with ICIs, a decrease in tumor metabolism indices as early as 8 weeks after therapy initiation occurred in responders. On the other hand, modifications of tumor burden indices occurred appreciably later. This time interval may be due to immune system reactivation and glucose consumption by tumor-infiltrated lymphocytes [24]. It seems that immune-related adverse events in immune cell therapy and ICIs have more impressive influence on 18F-FDG PET/CT scan, compared to the anti-CD20 immunotherapy. There are more anti-CD20 monoclonal antibody studies performed which allowed the analysis, while the current published studies in ICIs and cell therapy are quite heterogeneous with regard to response assessment and outcome prediction; therefore, dedicated analysis in this treatment cohort was not performed.
The number of enrolled papers on anti-CD20 monoclonal antibodies is quite different, compared to the other two categories: ICIs and cellular therapies. The latter ones have a small number of papers and do not have similar indices in all of them. This difference gives different strength to the results related to the anti-CD20 mAbs. With the support of the large number of studies on anti-CD20 mAbs, we could calculate pooled HRs, whereas meta-analysis and pooled ratio calculation were not possible for ICIs and cellular therapies. On the other hand, in the category of anti-CD20 mAbs, the depicted funnel plots showed asymmetry and probable publication bias in pooled HR of some of baseline parameters (MTV, TLG, and SUVmax for PFS and MTV for OS) as well as pooled HR of interim response assessment using DS for OS. This was the main limitation that we encountered in the present study.
Conclusion
Our present study revealed that with anti-CD20 therapy, baseline MTV on 18F-FDG PET/CT has the highest HRs for both PFS and OS. In response assessment of anti-CD20 therapy, Deauville score in early and late time points has higher HRs for PFS and OS compared to the international harmonization project criteria. While early changes in 18F-FDG PET parameters may be predictive of response to ICIs and cell therapy treatment, further studies are required to establish the optimal response parameters following treatment of lymphoma patients.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Vassilakopoulos TP, Chatzidimitriou C, Asimakopoulos JV, Arapaki M, Tzoras E, Angelopoulou MK, et al. Immunotherapy in Hodgkin lymphoma: present status and future strategies. Cancers (Basel). 2019;11(8):1071. https://doi.org/10.3390/cancers11081071.
Lopci E, Meignan M. Current evidence on PET response assessment to immunotherapy in lymphomas. PET Clin. 2020;15:23–34. https://doi.org/10.1016/j.cpet.2019.08.011.
Lin N, Song Y, Zhu J. Immune checkpoint inhibitors in malignant lymphoma: advances and perspectives. Chin J Cancer Res. 2020;32:303–18. https://doi.org/10.21147/j.issn.1000-9604.2020.03.03.
Rituximab (marketed as Rituxan) Information. U.S. Food and Drug Administration website. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/rituximab-marketed-rituxan-information. Updated July 23, 2015. Accessed Aug 28, 2021.
Obinutuzumab. U.S. Food and Drug Administration website. https://www.fda.gov/drugs/resources-information-approved-drugs/obinutuzumab. Updated Feb 26, 2016. Accessed Aug 28, 2021.
Zelenetz AD, Salles G, Mason KD, Casulo C, Le Gouill S, Sehn LH, et al. Venetoclax plus R- or G-CHOP in non-Hodgkin lymphoma: results from the CAVALLI phase 1b trial. Blood. 2019;133:1964–76. https://doi.org/10.1182/blood-2018-11-880526.
Pembrolizumab (Keytruda) 5–10–2017. U.S. Food and Drug Administration website. https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-5-10-2017. Updated May 11, 2017. Accessed Aug 28, 2021.
Nivolumab (Opdivo) for Hodgkin Lymphoma. U.S. Food and Drug Administration website. https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-opdivo-hodgkin-lymphoma. Updated May 17, 2016. Accessed Aug 28, 2021.
FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. U.S. Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-approves-car-t-cell-therapy-treat-adults-certain-types-large-b-cell-lymphoma#:~:text=The%20U.S.%20Food%20and%20Drug,two%20other%20kinds%20of%20treatment. Updated Mar 21, 2018. Accessed Aug 28, 2021.
McCarten KM, Nadel HR, Shulkin BL, Cho SY. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr Radiol. 2019;49:1545–64. https://doi.org/10.1007/s00247-019-04529-8.
Voltin CA, Mettler J, Grosse J, Dietlein M, Baues C, Schmitz C, et al. FDG-PET imaging for Hodgkin and diffuse large B-cell lymphoma-an updated overview. Cancers (Basel). 2020;12(3):601. https://doi.org/10.3390/cancers12030601.
Kirienko M, Sollini M, Chiti A. Hodgkin lymphoma and imaging in the era of anti-PD-1/PD-L1 therapy. Clinical and Translational Imaging. 2018;6:417–27.
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32:3059–68. https://doi.org/10.1200/JCO.2013.54.8800.
Cheson BD, Ansell S, Schwartz L, Gordon LI, Advani R, Jacene HA, et al. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood. 2016;128:2489–96. https://doi.org/10.1182/blood-2016-05-718528.
Gundogan C, Guzel Y, Can C, Alabalik U, Komek H. False-positive 68Ga-fibroblast activation protein-specific inhibitor uptake of benign lymphoid tissue in a patient with breast cancer. Clin Nucl Med. 2021;46:e433–5. https://doi.org/10.1097/RLU.0000000000003594.
Adams HJ, de Klerk JM, Fijnheer R, Heggelman BG, Dubois SV, Nievelstein RA, et al. Prognostic superiority of the National Comprehensive Cancer Network International Prognostic Index over pretreatment whole-body volumetric-metabolic FDG-PET/CT metrics in diffuse large B-cell lymphoma. Eur J Haematol. 2015;94:532–9. https://doi.org/10.1111/ejh.12467.
Allen PB, Savas H, Evens AM, Advani R, Palmer B, Pro B, et al. Pembrolizumab followed by AVD in untreated early unfavorable and advanced stage classical Hodgkin lymphoma. Blood 2020.
Annunziata S, Cuccaro A, Tisi MC, Hohaus S, Rufini V. FDG-PET/CT at the end of immuno-chemotherapy in follicular lymphoma: the prognostic role of the ratio between target lesion and liver SUVmax (rPET). Ann Nucl Med. 2018;32:372–7. https://doi.org/10.1007/s12149-018-1243-2.
Bartlett NL, Herrera AF, Domingo-Domenech E, Mehta A, Forero-Torres A, Garcia-Sanz R, et al. A phase 1b study of AFM13 in combination with pembrolizumab in patients with relapsed or refractory Hodgkin lymphoma. Blood, J Am Soc Hematol. 2020;136:2401–9.
Baudard M, Comte F, Conge AM, Mariano-Goulart D, Klein B, Rossi JF. Importance of [18F]fluorodeoxyglucose-positron emission tomography scanning for the monitoring of responses to immunotherapy in follicular lymphoma. Leuk Lymphoma. 2007;48:381–8. https://doi.org/10.1080/10428190601094354.
Casasnovas R-O, Ysebaert L, Thieblemont C, Bachy E, Feugier P, Delmer A, et al. FDG-PET–driven consolidation strategy in diffuse large B-cell lymphoma: final results of a randomized phase 2 study. Blood, J Am Soc Hematol. 2017;130:1315–26.
Casasnovas R-O, Meignan M, Berriolo-Riedinger A, Bardet S, Julian A, Thieblemont C, et al. SUVmax reduction improves early prognosis value of interim positron emission tomography scans in diffuse large B-cell lymphoma. Blood, J Am Soc Hematol. 2011;118:37–43.
Cashen AF, Dehdashti F, Luo J, Homb A, Siegel BA, Bartlett NL. 18F-FDG PET/CT for early response assessment in diffuse large B-cell lymphoma: poor predictive value of international harmonization project interpretation. J Nucl Med. 2011;52:386–92. https://doi.org/10.2967/jnumed.110.082586.
Castello A, Grizzi F, Qehajaj D, Rahal D, Lutman F, Lopci E. (18)F-FDG PET/CT for response assessment in Hodgkin lymphoma undergoing immunotherapy with checkpoint inhibitors. Leuk Lymphoma. 2019;60:367–75. https://doi.org/10.1080/10428194.2018.1488254.
Ceriani L, Gritti G, Cascione L. baseline metabolic heterogeneity and metabolic tumor volume in DLBCL prognostic model. Blood Adv. 2020;4(6):1082–92.
Ceriani L, Martelli M, Gospodarowicz MK, Ricardi U, Ferreri AJ, Chiappella A, et al. Positron emission tomography/computed tomography assessment after immunochemotherapy and irradiation using the Lugano classification criteria in the IELSG-26 study of primary mediastinal B-cell lymphoma. Int J Radiat Oncol Biol Phys. 2017;97:42–9.
Chang CC, Cho SF, Chuang YW, Lin CY, Chang SM, Hsu WL, et al. Prognostic significance of total metabolic tumor volume on (18)F-fluorodeoxyglucose positron emission tomography/ computed tomography in patients with diffuse large B-cell lymphoma receiving rituximab-containing chemotherapy. Oncotarget. 2017;8:99587–600. https://doi.org/10.18632/oncotarget.20447.
Chen A, Mokrane FZ, Schwartz L, Morschhauser F, Stamatoullas A, Schiano de Colella JM, et al. Early (18)F-FDG PET/CT response predicts survival in relapsed/refractory Hodgkin lymphoma treated with nivolumab. J Nucl Med. 2019 https://doi.org/10.2967/jnumed.119.232827.
Chen Y, Zhou M, Liu J, Huang G. Prognostic value of bone marrow FDG uptake pattern of PET/CT in newly diagnosed diffuse large B-cell lymphoma. J Cancer. 2018;9:1231.
Chen-Liang TH, Martín-Santos T, Jerez A, Rodríguez-García G, Senent L, Martínez-Millán C, et al. Bone marrow biopsy superiority over PET/CT in predicting progression-free survival in a homogeneously-treated cohort of diffuse large B-cell lymphoma. Cancer Med. 2017;6:2507–14.
Cottereau AS, Nioche C, Dirand AS, Clerc J, Morschhauser F, Casasnovas O, et al. (18)F-FDG PET dissemination features in diffuse large B-cell lymphoma are predictive of outcome. J Nucl Med. 2020;61:40–5. https://doi.org/10.2967/jnumed.119.229450.
Cottereau AS, Lanic H, Mareschal S, Meignan M, Vera P, Tilly H, et al. Molecular profile and FDG-PET/CT total metabolic tumor volume improve risk classification at diagnosis for patients with diffuse large B-cell lymphoma. Clin Cancer Res. 2016;22:3801–9. https://doi.org/10.1158/1078-0432.CCR-15-2825.
Cui Q, Vari F, Cristino AS, Salomon C, Rice GE, Sabdia MB, et al. Circulating cell-free miR-494 and miR-21 are disease response biomarkers associated with interim-positron emission tomography response in patients with diffuse large B-cell lymphoma. Oncotarget. 2018;9:34644.
Delfau-Larue M-H, van der Gucht A, Dupuis J, Jais J-P, Nel I, Beldi-Ferchiou A, et al. Total metabolic tumor volume, circulating tumor cells, cell-free DNA: distinct prognostic value in follicular lymphoma. Blood Adv. 2018;2:807–16.
de Oliveira Costa R, Neto AH, Siqueira S, Lage LAdPC, de Paula HM, Coutinho AM, et al. Interim fluorine-18 fluorodeoxyglucose PET-computed tomography and cell of origin by immunohistochemistry predicts progression-free and overall survival in diffuse large B-cell lymphoma patients in the rituximab era. Nucl Med Commun. 2016;37:1095.
Dercle L, Seban RD, Lazarovici J, Schwartz LH, Houot R, Ammari S, et al. (18)F-FDG PET and CT scans detect new imaging patterns of response and progression in patients with Hodgkin lymphoma treated by anti-programmed death 1 immune checkpoint inhibitor. J Nucl Med. 2018;59:15–24. https://doi.org/10.2967/jnumed.117.193011.
Derlin T, Schultze-Florey C, Werner RA, Möhn N, Skripuletz T, David S, et al. 18 F-FDG PET/CT of off-target lymphoid organs in CD19-targeting chimeric antigen receptor T-cell therapy for relapsed or refractory diffuse large B-cell lymphoma. Ann Nucl Med. 2021;35:132–8.
Dührsen U, Müller S, Hertenstein B, Thomssen H, rg Kotzerke J, Mesters R, et al. Positron emission tomography-guided therapy of aggressive non-Hodgkin lymphomas PETAL: a multicenter, randomized phase III trial. J Clin Oncol. 2018;36:2024–34.
Dunleavy K, Fanale MA, Abramson JS, Noy A, Caimi PF, Pittaluga S, et al. A prospective multicenter phase 2 study of dose-adjusted-EPOCH-R in untreated MYC-rearranged aggressive B-cell lymphoma. The Lancet Haematology. 2018;5:e609.
El-Galaly TC, Villa D, Michaelsen TY, Hutchings M, Mikhaeel NG, Savage KJ, et al. The number of extranodal sites assessed by PET/CT scan is a powerful predictor of CNS relapse for patients with diffuse large B-cell lymphoma: an international multicenter study of 1532 patients treated with chemoimmunotherapy. Eur J Cancer. 2017;75:195–203.
El-Galaly TC, Villa D, Alzahrani M, Hansen JW, Sehn LH, Wilson D, et al. Outcome prediction by extranodal involvement, IPI, R-IPI, and NCCN-IPI in the PET/CT and rituximab era: AD anish–C anadian study of 443 patients with diffuse-large B-cell lymphoma. Am J Hematol. 2015;90:1041–6.
Esfahani SA, Heidari P, Halpern EF, Hochberg EP, Palmer EL, Mahmood U. Baseline total lesion glycolysis measured with 18F-FDG PET/CT as a predictor of progression-free survival in diffuse large B-cell lymphoma: a pilot study. Am J Nucl Med Mol Imaging. 2013;3:272.
Gallicchio R, Mansueto G, Simeon V, Nardelli A, Guariglia R, Capacchione D, et al. F-18 FDG PET/CT quantization parameters as predictors of outcome in patients with diffuse large B-cell lymphoma. Eur J Haematol. 2014;92:382–9. https://doi.org/10.1111/ejh.12268.
Giulino-Roth L, O’Donohue T, Chen Z, Bartlett NL, LaCasce A, Martin-Doyle W, et al. Outcomes of adults and children with primary mediastinal B-cell lymphoma treated with dose-adjusted EPOCH-R. Br J Haematol. 2017;179:739–47.
Han EJ, Joo Hyun O, Yoon H, Jung SE, Park G, Choi BO, et al. FDG PET/CT response in diffuse large B-cell lymphoma: reader variability and association with clinical outcome. Medicine. 2016;95.
Han HS, Escalon MP, Hsiao B, Serafini A, Lossos IS. High incidence of false-positive PET scans in patients with aggressive non-Hodgkin’s lymphoma treated with rituximab-containing regimens. Ann Oncol. 2009;20:309–18. https://doi.org/10.1093/annonc/mdn629.
Hart D, Avivi I, Thomson K, Peggs K, Morris E, Goldstone A, et al. Use of 18F-FDG positron emission tomography following allogeneic transplantation to guide adoptive immunotherapy with donor lymphocyte infusions. Br J Haematol. 2005;128:824–9.
Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013;40:1312–20.
Jeon YW, O JH, Park KS, Min GJ, Park SS, Yoon JH, et al. Prognostic impact of interim positron emission tomography in mantle cell lymphoma patients treated with frontline R-CHOP. Bri J Haematol. 2020;188:860–71.
Khan AB, Barrington SF, Mikhaeel NG, Hunt AA, Cameron L, Morris T, et al. PET-CT staging of DLBCL accurately identifies and provides new insight into the clinical significance of bone marrow involvement. Blood. 2013;122:61–7.
Kim SJ, Yi HK, Lim CH, Cho YS, Choi JY, Choe YS, et al. Intra-patient variability of FDG standardized uptake values in mediastinal blood pool, liver, and myocardium during R-CHOP chemotherapy in patients with diffuse large B-cell lymphoma. Nucl Med Mol Imaging. 2016;50:300–7. https://doi.org/10.1007/s13139-016-0432-y.
Kim J, Hong J, Kim SG, Hwang KH, Kim M, Ahn HK, et al. Prognostic value of metabolic tumor volume estimated by 18 F-FDG positron emission tomography/computed tomography in patients with diffuse large B-cell lymphoma of stage II or III disease. Nucl Med Mol Imaging. 2014;48:187–95.
Kim TM, Paeng JC, Chun IK, Keam B, Jeon YK, Lee SH, et al. Total lesion glycolysis in positron emission tomography is a better predictor of outcome than the International Prognostic Index for patients with diffuse large B cell lymphoma. Cancer. 2013;119:1195–202. https://doi.org/10.1002/cncr.27855.
Kitajima K, Okada M, Yoshihara K, Tokugawa T, Sawada A, Yoshihara S, et al. Predictive value of interim FDG-PET/CT findings in patients with diffuse large B-cell lymphoma treated with R-CHOP. Oncotarget. 2019;10:5403–11. https://doi.org/10.18632/oncotarget.27103.
Kocurek A, Malkowski B, Giza A, Jurczak W. Primary mediastinal B-cell lymphoma - metabolic and anatomical features in 18FDG-PET/CT and response to therapy. Contemp Oncol (Pozn). 2016;20:297–301. https://doi.org/10.5114/wo.2016.61849.
Kong Y, Qu L, Li Y, Liu D, Lv X, Han J. Predictive significance of a new prognostic score for patients with diffuse large B-cell lymphoma in the interim-positron emission tomography findings. Med. 2016;95:e2808.
Kostakoglu L, Martelli M, Sehn LH, Belada D, Carella A-M, Chua N, et al. End-of-treatment PET/CT predicts PFS and OS in DLBCL after first-line treatment: results from GOYA. Blood Adv. 2021;5:1283–90.
Lepik KV, Fedorova LV, Kondakova EV, Zalyalov YR, Babenko EV, Lepik EE, et al. A phase 2 study of nivolumab using a fixed dose of 40 mg (Nivo40) in patients with relapsed/refractory Hodgkin lymphoma. HemaSphere. 2020;4:e480.
Leppä S, Jørgensen J, Tierens A, Meriranta L, Østlie I, de Nully BP, et al. Patients with high-risk DLBCL benefit from dose-dense immunochemotherapy combined with early systemic CNS prophylaxis. Blood Adv. 2020;4:1906–15.
Mamot C, Klingbiel D, Hitz F, Renner C, Pabst T, Driessen C, et al. Final results of a prospective evaluation of the predictive value of interim positron emission tomography in patients with diffuse large B-cell lymphoma treated with R-CHOP-14 (SAKK 38/07). J Clin Oncol. 2015;33:2523–9.
Mayerhoefer ME, Staudenherz A, Kiesewetter B, Weber M, Simonitsch-Klupp I, Gibbs P, et al. Pre-therapeutic total lesion glycolysis on [(18)F]FDG-PET enables prognostication of 2-year progression-free survival in MALT lymphoma patients treated with CD20-antibody-based immunotherapy. Mol Imaging Biol. 2019;21(6):1192. https://doi.org/10.1007/s11307-019-01329-2.
Mayerhoefer ME, Riedl CC, Kumar A, Gibbs P, Weber M, Tal I, et al. Radiomic features of glucose metabolism enable prediction of outcome in mantle cell lymphoma. Eur J Nucl Med Mol Imaging. 2019;46:2760–9. https://doi.org/10.1007/s00259-019-04420-6.
Mayerhoefer ME, Raderer M, Jaeger U, Staber P, Kiesewetter B, Senn D, et al. Ultra-early response assessment in lymphoma treatment: [(18)F]FDG PET/MR captures changes in glucose metabolism and cell density within the first 72 hours of treatment. Eur J Nucl Med Mol Imaging. 2018;45:931–40. https://doi.org/10.1007/s00259-018-3937-z.
Mayerhoefer ME, Karanikas G, Kletter K, Kiesewetter B, Weber M, Rausch I, et al. Can interim 18F-FDG PET or diffusion-weighted MRI predict end-of-treatment outcome in FDG-avid MALT lymphoma after rituximab-based therapy? A preliminary study in 15 patients. Clin Nucl Med. 2016;41:837–43. https://doi.org/10.1097/RLU.0000000000001395.
Melani C, Advani R, Roschewski M, Walters KM, Chen CC, Baratto L, et al. End-of-treatment and serial PET imaging in primary mediastinal B-cell lymphoma following dose-adjusted EPOCH-R: a paradigm shift in clinical decision making. Haematologica. 2018;103:1337–44. https://doi.org/10.3324/haematol.2018.192492.
Micallef IN, Maurer MJ, Wiseman GA, Nikcevich DA, Kurtin PJ, Cannon MW, et al. Epratuzumab with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy in patients with previously untreated diffuse large B-cell lymphoma. Blood, J Am Soc Hematol. 2011;118:4053–61.
Mikhaeel NG, Smith D, Dunn JT, Phillips M, Moller H, Fields PA, et al. Combination of baseline metabolic tumour volume and early response on PET/CT improves progression-free survival prediction in DLBCL. Eur J Nucl Med Mol Imaging. 2016;43:1209–19. https://doi.org/10.1007/s00259-016-3315-7.
Minamimoto R, Fayad L, Advani R, Vose J, Macapinlac H, Meza J, et al. Diffuse large B-cell lymphoma: prospective multicenter comparison of early interim FLT PET/CT versus FDG PET/CT with IHP, EORTC, Deauville, and PERCIST criteria for early therapeutic monitoring. Radiology. 2016;280:220–9.
Mir F, Barrington SF, Brown H, Nielsen T, Sahin D, Meignan M, et al. Baseline SUVmax did not predict histological transformation in follicular lymphoma in the phase 3 GALLIUM study. Blood. 2020;135:1214–8. https://doi.org/10.1182/blood.2019001091.
Mokrane F-Z, Chen A, Schwartz LH, Morschhauser F, Stamatoullas A, Schiano de Colella J-M, et al. Performance of CT compared with 18F-FDG PET in predicting the efficacy of nivolumab in relapsed or refractory Hodgkin lymphoma. Radiology. 2020;295:651–61.
Morschhauser F, Feugier P, Flinn IW, Gasiorowski R, Greil R, Illés Á, et al. A phase 2 study of venetoclax plus R-CHOP as first-line treatment for patients with diffuse large B-cell lymphoma. Blood, J Am Soc Hematol. 2021;137:600–9.
Moskowitz CH, Schöder H, Teruya-Feldstein J, Sima C, Iasonos A, Portlock CS, et al. Risk-adapted dose-dense immunochemotherapy determined by interim FDG-PET in advanced-stage diffuse large B-cell lymphoma. J Clin Oncol. 2010;28:1896.
Oñate-Ocaña LF, Cortés V, Castillo-Llanos R, Terrazas A, Garcia-Perez O, Pitalúa-Cortes Q, et al. Metabolic tumor volume changes assessed by interval 18fluorodeoxyglucose positron emission tomography-computed tomography for the prediction of complete response and survival in patients with diffuse large B-cell lymphoma. Oncol Lett. 2018;16:1411–8.
Pinnix CC, Ng AK, Dabaja BS, Milgrom SA, Gunther JR, Fuller CD, et al. Positron emission tomography–computed tomography predictors of progression after DA-R-EPOCH for PMBCL. Blood Adv. 2018;2:1334–43.
Rutherford SC, Herold M, Hiddemann W, Kostakoglu L, Marcus R, Martelli M, et al. Impact of bone marrow biopsy on response assessment in immunochemotherapy-treated lymphoma patients in GALLIUM and GOYA. Blood Adv. 2020;4:1589–93. https://doi.org/10.1182/bloodadvances.2019001261.
Sasanelli M, Meignan M, Haioun C, Berriolo-Riedinger A, Casasnovas RO, Biggi A, et al. Pretherapy metabolic tumour volume is an independent predictor of outcome in patients with diffuse large B-cell lymphoma. Eur J Nucl Med Mol Imaging. 2014;41:2017–22. https://doi.org/10.1007/s00259-014-2822-7.
Schmitz C, Hüttmann A, Müller SP, Hanoun M, Boellaard R, Brinkmann M, et al. Dynamic risk assessment based on positron emission tomography scanning in diffuse large B-cell lymphoma: post-hoc analysis from the PETAL trial. Eur J Cancer. 2020;124:25–36.
Senjo H, Hirata K, Izumiyama K, Minauchi K, Tsukamoto E, Itoh K, et al. High metabolic heterogeneity on baseline 18FDG-PET/CT scan as a poor prognostic factor for newly diagnosed diffuse large B-cell lymphoma. Blood Adv. 2020;4:2286–96.
Shah NN, Nagle SJ, Torigian DA, Farwell MD, Hwang WT, Frey N, et al. Early positron emission tomography/computed tomography as a predictor of response after CTL019 chimeric antigen receptor -T-cell therapy in B-cell non-Hodgkin lymphomas. Cytotherapy. 2018;20:1415–8. https://doi.org/10.1016/j.jcyt.2018.10.003.
Song G-Y, Yoon SE, Kim SJ, Kim JS, Koh Y, Moon J-H, et al. Prognostic significance of interim PET/CT response for the treatment of advanced-stage marginal zone lymphoma in the post-rituximab era. Sci Rep. 2020;10:1–8.
Song MK, Yang DH, Lee GW, Lim SN, Shin S, Pak KJ, et al. High total metabolic tumor volume in PET/CT predicts worse prognosis in diffuse large B cell lymphoma patients with bone marrow involvement in rituximab era. Leuk Res. 2016;42:1–6. https://doi.org/10.1016/j.leukres.2016.01.010.
Song YS, Lee WW, Lee JS, Kim SE. Prediction of central nervous system relapse of diffuse large B-cell lymphoma using pretherapeutic [18F] 2-fluoro-2-deoxyglucose (FDG) positron emission tomography/computed tomography. Medicine. 2015;94:e1978.
Song MK, Chung JS, Shin HJ, Lee SM, Lee SE, Lee HS, et al. Clinical significance of metabolic tumor volume by PET/CT in stages II and III of diffuse large B cell lymphoma without extranodal site involvement. Ann Hematol. 2012;91:697–703. https://doi.org/10.1007/s00277-011-1357-2.
Song MK, Chung JS, Shin HJ, Moon JH, Lee JO, Lee HS, et al. Prognostic value of metabolic tumor volume on PET / CT in primary gastrointestinal diffuse large B cell lymphoma. Cancer Sci. 2012;103:477–82. https://doi.org/10.1111/j.1349-7006.2011.02164.x.
Sun H, Yu Z, Ma N, Zhou J, Tian R, Zhao M, et al. Risk stratification of diffuse large B-cell lymphoma with interim PET/CT by combining Deauville scores and International Prognostic Index. Cancer Manag Res. 2019;11:9449–57. https://doi.org/10.2147/CMAR.S218678.
Swinnen LJ, Li H, Quon A, Gascoyne R, Hong F, Ranheim EA, et al. Response-adapted therapy for aggressive non-Hodgkin’s lymphomas based on early [18F] FDG-PET scanning: ECOG-ACRIN Cancer Research Group study (E3404). Br J Haematol. 2015;170:56–65.
Takasaki H, Yamamoto W, Ishii Y, Takahashi H, Watanabe R, Harada T, et al. Post-treatment PET–CT findings may predict the prognosis of DLBCL with a bulky mass. Indian Journal of Hematology and Blood Transfusion. 2015;31:346–51.
Tateishi U, Tatsumi M, Terauchi T, Ando K, Niitsu N, Kim WS, et al. Prognostic significance of metabolic tumor burden by positron emission tomography/computed tomography in patients with relapsed/refractory diffuse large B-cell lymphoma. Cancer Sci. 2015;106:186–93.
Toledano MN, Vera P, Tilly H, Jardin F, Becker S. Comparison of therapeutic evaluation criteria in FDG-PET/CT in patients with diffuse large-cell B-cell lymphoma: Prognostic impact of tumor/liver ratio. PLoS ONE. 2019;14:e0211649. https://doi.org/10.1371/journal.pone.0211649.
Trotman J, Barrington SF, Belada D, Meignan M, MacEwan R, Owen C, et al. Prognostic value of end-of-induction PET response after first-line immunochemotherapy for follicular lymphoma (GALLIUM): secondary analysis of a randomised, phase 3 trial. Lancet Oncol. 2018;19:1530–42. https://doi.org/10.1016/S1470-2045(18)30618-1.
Vaxman I, Bernstine H, Kleinstern G, Hendin N, Shimony S, Domachevsky L, et al. FDG PET/CT as a diagnostic and prognostic tool for the evaluation of marginal zone lymphoma. Hematol Oncol. 2019;37:168–75. https://doi.org/10.1002/hon.2578.
Vercellino L, Cottereau A-S, Casasnovas O, Tilly H, Feugier P, Chartier L, et al. High total metabolic tumor volume at baseline predicts survival independent of response to therapy. Blood, J Am Soc Hematol. 2020;135:1396–405.
Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D’Aveni-Piney M, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4:5607–15.
Voltin C-A, Mettler J, Mueller H, Fuchs M, Baues C, Dietlein M, et al. Metabolic tumor volume for early response assessment in early-stage unfavorable Hodgkin lymphoma treated with nivolumab in the GHSG Nivahl phase II trial. American Society of Hematology Washington, DC; 2019.
Wang J, Hu Y, Yang S, Wei G, Zhao X, Wu W, et al. Role of fluorodeoxyglucose positron emission tomography/computed tomography in predicting the adverse effects of chimeric antigen receptor T cell therapy in patients with non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2019;25:1092–8.
Wang R, Xu B, Liu C, Guan Z, Zhang J, Li F, et al. Prognostic value of interim fluorodeoxyglucose and fluorothymidine PET/CT in diffuse large B-cell lymphoma. Br J Radiol. 2018;91:20180240.
Wei Z, Li J, Cheng Z, Yuan L, Liu P. A single center experience: rituximab plus cladribine is an effective and safe first-line therapy for unresectable bronchial-associated lymphoid tissue lymphoma. J Thorac Dis. 2017;9:1081–92. https://doi.org/10.21037/jtd.2017.03.81.
Wong-Sefidan I, Byrtek M, Zhou X, Friedberg JW, Flowers CR, Zelenetz AD, et al. [18F] Positron emission tomography response after rituximab-containing induction therapy in follicular lymphoma is an independent predictor of survival after adjustment for FLIPI in academic and community-based practice. Leuk Lymphoma. 2017;58:809–15.
Yim SK, Yhim H-Y, Han Y-H, Jeon S-Y, Lee N-R, Song E-K, et al. Early risk stratification for diffuse large B-cell lymphoma integrating interim Deauville score and International Prognostic Index. Ann Hematol. 2019;98:2739–48.
Younes A, Oki Y, McLaughlin P, Copeland AR, Goy A, Pro B, et al. Phase 2 study of rituximab plus ABVD in patients with newly diagnosed classical Hodgkin lymphoma. Blood, J Am Soc Hematol. 2012;119:4123–8.
Zhang YY, Song L, Zhao MX, Hu K. A better prediction of progression-free survival in diffuse large B-cell lymphoma by a prognostic model consisting of baseline TLG and %DeltaSUVmax. Cancer Med. 2019;8:5137–47. https://doi.org/10.1002/cam4.2284.
Zhang X, Fan W, Xia Z-J, Hu Y-Y, Lin X-P, Zhang Y-R, et al. Use of subsequent PET/CT in diffuse large B-cell lymphoma patients in complete remission following primary therapy. Chin J Cancer. 2015;34:70–8.
Zhao P, Yu T, Pan Z. Prognostic value of the baseline 18F-FDG PET/CT metabolic tumour volume (MTV) and further stratification in low-intermediate (LI) and high-intermediate (HI) risk NCCNIPI subgroup by MTV in DLBCL MTV predict prognosis in DLBCL. Ann Nucl Med. 2021;35:24–30.
Zhou Y, Zhao Z, Li J, Zhang B, Sang S, Wu Y, et al. Prognostic values of baseline, interim and end-of therapy (18)F-FDG PET/CT in patients with follicular lymphoma. Cancer Manag Res. 2019;11:6871–85. https://doi.org/10.2147/CMAR.S216445.
Zhou M, Chen Y, Huang H, Zhou X, Liu J, Huang G. Prognostic value of total lesion glycolysis of baseline 18F-fluorodeoxyglucose positron emission tomography/computed tomography in diffuse large B-cell lymphoma. Oncotarget. 2016;7:83544–53. https://doi.org/10.18632/oncotarget.13180.
Zinzani PL, Gandolfi L, Broccoli A, Argnani L, Fanti S, Pellegrini C, et al. Midtreatment 18F-fluorodeoxyglucose positron-emission tomography in aggressive non-Hodgkin lymphoma. Cancer. 2011;117:1010–8. https://doi.org/10.1002/cncr.25579.
Moher D, Liberati A, Tetzlaff J, Group P. Altman DG Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS medicine. 2009;6:e1000097.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Zahra Kiamanesh, Narjess Ayati, and Ramin Sadeghi. The first draft of the manuscript was written by Zahra Kiamanesh and Narjess Ayati and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
This is a review study with no ethical approval is required.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—General.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kiamanesh, Z., Ayati, N., Sadeghi, R. et al. The value of FDG PET/CT imaging in outcome prediction and response assessment of lymphoma patients treated with immunotherapy: a meta-analysis and systematic review. Eur J Nucl Med Mol Imaging 49, 4661–4676 (2022). https://doi.org/10.1007/s00259-022-05918-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-022-05918-2