Abstract
Purpose
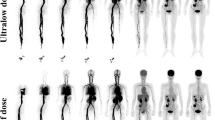
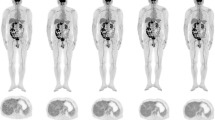
This study was to evaluate the effects of an ultra-low dose of [18F]-FDG on the image quality of total-body PET/CT and its lesion detectability in colorectal cancer (CRC).
Methods
Sixty-two CRC patients who underwent total-body PET/CT (uEXPLORER, United Imaging Healthcare, Shanghai, China) with an ultra-low dose (0.37 MBq/kg) of [18F]-FDG were enrolled in this retrospective study. The PET images were reconstructed with the entire 15-min dataset first and then split into 13-, 8-, 5-, 4-, 3-, 2-, and 1-min duration groups to simulate fast scanning images. For simplicity, the images reconstructed with the data from 15 to 1 min were referred to as G15, G13, and so on until G1. Subjective image quality was assessed with 5-point Likert scales. The objective image quality parameters included the SUVmax, SUVmean, and signal-to-noise ratio (SNR) of the liver and blood pool and the SUVmax and tumor-to-background ratio (TBR) of the lesions. G15 served as the control to evaluate lesion detectability.
Results
A total of 62 patients (43 men, 19 women; age 41–88, mean ± SD 64.0 ± 10.9 years) with 64 CRC primary tumor lesions and 10 low-grade intraepithelial neoplasia (LGIN) lesions were enrolled in this study. The subjective scores were highest for G15 (4.5 ± 0.5) and then decreased from G13 (4.3 ± 0.4) to G8 (3.7 ± 0.5). The liver SNR increased with the extension of acquisition time from G8 (17.2 ± 2.8) to G13 (20.6 ± 3.4) and G15 (21.9 ± 3.4). The liver SNR of G8 was not significantly different from that of G13 (p = 0.15) and was significantly different from that of G15 (p = 0.001). All 64 CRC lesions could be identified in all image groups, even on G1. One of ten LGINs was missed on G1, G2, and G3, and one LGIN was missed on G1, G2, G3, and G4. G15 served as the control, and 100% (48/48) lymph nodes could be found on G13 and G8 compared to 93.8% (45/48) lymph nodes on G5 and G4, 85.4% (41/48) lymph nodes on G3, 81.3% (39/48) lymph nodes on G2, and 77.1% (37/48) lymph nodes on G1. For liver metastases, there were no missed liver lesions on G13 and G8 and 3, 4, 6, 7, and 9 missed liver lesions on G5, G4, G3, G2, and G1, respectively. For other areas of metastasis, including the lung, peritoneum, and ovaries, there were no missed lesions in any group.
Conclusions
Total-body PET/CT with an ultra-low dose of [18F]-FDG can maintain satisfactory image quality and lesion detectability in CRC.





Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(3):329–59.
Rice SR, Chuong M, Koroulakis A, et al. The utility of PET/computed tomography for radiation oncology planning, surveillance, and prognosis prediction of gastrointestinal tumors. PET Clin. 2020;15(1):77–87.
Unterrainer M, Eze C, Ilhan H, et al. Recent advances of PET imaging in clinical radiation oncology. Radiat Oncol. 2020;15(1):88.
Bulens P, Thomas M, Deroose CM, et al. PET imaging in adaptive radiotherapy of gastrointestinal tumors. Q J Nucl Med Mol Imaging. 2018;62(4):385–403.
Rodríguez-Fraile M, Cózar-Santiago MP, Sabaté-Llobera A, et al. FDG PET/CT in colorectal cancer. Rev Esp Med Nucl Imagen Mol. 2020;39(1):57–66.
Zhang X, Xie Z, Berg E, et al. Total-Body dynamic reconstruction and parametric imaging on the uEXPLORER. J Nucl Med. 2020;61(2):285–91.
Zhang X, Cherry SR, Xie Z, et al. Subsecond total-body imaging using ultrasensitive positron emission tomography. Proc Natl Acad Sci USA. 2020;117(5):2265–7.
Spencer BA, Berg E, Schmall JP, et al. Performance evaluation of the uEXPLORER Total-body PET/CT scanner based on NEMA NU 2–2018 with additional tests to characterize long axial field-of-view PET scanners. J Nucl Med. 2021;62(6):861–70.
Badawi RD, Shi H, Hu P, et al. First human imaging studies with the EXPLORER Total-body PET Scanner. J Nucl Med. 2019;60(3):299–303.
Zhang YQ, Hu PC, Wu RZ, et al. The image quality, lesion detectability, and acquisition time of (18)F-FDG total-body PET/CT in oncological patients. Eur J Nucl Med Mol Imaging. 2020;47(11):2507–15.
Tan H, Sui X, Yin H, et al. Total-body PET/CT using half-dose FDG and compared with conventional PET/CT using full-dose FDG in lung cancer. Eur J Nucl Med Mol Imaging. 2021;48(6):1966–75.
Liu G, Xu H, Hu P, et al. Kinetic metrics of (18)F-FDG in normal human organs identified by systematic dynamic total-body positron emission tomography. Eur J Nucl Med Mol Imaging. 2021;48(8):2363–72.
Liu G, Hu P, Yu H, et al. Ultra-low-activity total-body dynamic PET imaging allows equal performance to full-activity PET imaging for investigating kinetic metrics of (18)F-FDG in healthy volunteers. Eur J Nucl Med Mol Imaging. 2021;48(8):2373–83.
Lu YY, Chen JH, Ding HJ, et al. A systematic review and meta-analysis of pretherapeutic lymph node staging of colorectal cancer by 18F-FDG PET or PET/CT. Nucl Med Commun. 2012;33(11):1127–33.
Tsili AC, Alexiou G, Naka C, Argyropoulou MI. Imaging of colorectal cancer liver metastases using contrast-enhanced US, multidetector CT, MRI, and FDG PET/CT: a meta-analysis. Acta Radiol. 2021;62(3):302–12.
Boellaard R, Delgado-Bolton R, Oyen WJ, et al. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42(2):328–54.
Van Sluis J, Boellaard R, Somasundaram A, et al. Image quality and semiquantitative measurements on the biograph vision PET/CT system: initial experiences and comparison with the biograph mCT. J Nucl Med. 2020;61(1):129–35.
Zhang X, Zhou J, Cherry SR, et al. Quantitative image reconstruction for total-body PET imaging using the 2-meter long EXPLORER scanner. Phys Med Biol. 2017;62(6):2465–85.
Cherry SR, Jones T, Karp JS, et al. Total-body PET: maximizing sensitivity to create new opportunities for clinical research and patient care. J Nucl Med. 2018;59(1):3–12.
Tan H, Gu Y, Yu H, et al. Total-Body PET/CT: current applications and future perspectives. Am J Roentgenol. 2020;215(2):325–37.
Pan T, Einstein SA, Kappadath SC, et al. Performance evaluation of the 5-Ring GE Discovery MI PET/CT system using the national electrical manufacturers association NU 2–2012 Standard. Med Phys. 2019;46(7):3025–33.
Akamatsu G, Ishikawa K, Mitsumoto K, et al. Improvement in PET/CT image quality with a combination of point-spread function and time-of-flight in relation to reconstruction parameters. J Nucl Med. 2012;53(11):1716–22.
Halpern BS, Dahlbom M, Quon A, et al. Impact of patient weight and emission scan duration on PET/CT image quality and lesion detectability. J Nucl Med. 2004;45(5):797–801.
Lee JY, Yoon SM, Kim JT, et al. Diagnostic and prognostic value of preoperative (18)F-fluorodeoxyglucose positron emission tomography/computed tomography for colorectal cancer: comparison with conventional computed tomography. Intest Res. 2017;15(2):208–14.
Yang Z, Liu Z. The efficacy of (18)F-FDG PET/CT-based diagnostic model in the diagnosis of colorectal cancer regional lymph node metastasis. Saudi J Biol Sci. 2020;27(3):805–11.
Kantorová I, Lipská L, Bêlohlávek O, et al. Routine (18)F-FDG PET preoperative staging of colorectal cancer: comparison with conventional staging and its impact on treatment decision making. J Nucl Med. 2003;44(11):1784–8.
Marashdeh WM, Al-Mugbel KM, Alebbini MM, et al. Pitfalls and value of organ specific approach in evaluating indeterminate lesions detected on CT in colorectal cancer by [F18] FDG PET/CT. Eur J Radiol Open. 2020;7:100264.
Kwak JY, Kim JS, Kim HJ, et al. Diagnostic value of FDG-PET/CT for lymph node metastasis of colorectal cancer. World J Surg. 2012;36(8):1898–905.
Funding
This study was supported by the National Science Foundation for Scholars of China (No. 81901796), the Shanghai Sailing Program Supported by Shanghai Science and Technology Commission (No. 19YF1408300), Clinical Research Plan of Shanghai Hospital Development Center (No. SHDC2020CR3079B), and the Shanghai Science and Technology Committee (No. 20DZ2201800), Special Fund for Clinical Research, Young Program of Zhongshan Hospital of Fudan University (No. 2018ZSQN38, 2019ZSYQ28, 2020ZSLC63), the Shanghai “Rising Stars of Medical Talent”-Youth Development Program (No. HWJRS2019-720), and the Shanghai Municipal Key Clinical Specialty Project (No. SHSLCZDZK03401). Next Generation Information Infrastructure Construction Project (No. 201901014). Collaborative Innovation Center for Molecular Imaging Precision Medicine, Shanxi Medical University, Taiyuan, Shanxi, 030001, People’s Republic of China.
Author information
Authors and Affiliations
Contributions
Hui Tan and Danjie Cai were involved in the study design, data analysis, and manuscript preparation. Xiuli Sui, Chi Qi, Guobing Liu, and Wujian Mao helped with data processing. Haojun Yu and Shuguang Chen helped with image acquisition and processing. Yiqiu Zhang and Pengcheng Hu helped with revision of the manuscript. Jianying Gu and Hongcheng Shi designed the study and contributed to the data analysis and writing of the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology—Digestive tract
Rights and permissions
About this article
Cite this article
Tan, H., Cai, D., Sui, X. et al. Investigating ultra-low-dose total-body [18F]-FDG PET/CT in colorectal cancer: initial experience. Eur J Nucl Med Mol Imaging 49, 1002–1011 (2022). https://doi.org/10.1007/s00259-021-05537-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05537-3