Abstract
Purpose
Clinical measurement of myocardial blood flow (MBF) has emerged as an important component of routine PET-CT assessment of myocardial perfusion in patients with known or suspected coronary artery disease. Although multiple society guidelines recommend patient-specific dosing, there is a lack of studies evaluating the efficacy of patient-specific dosing for quantitative MBF accuracy.
Methods
Two patient-specific dosing protocols (weight- and BMI-adjusted) were retrospectively evaluated in 435 consecutive clinical patients referred for PET myocardial perfusion assessment. MBF was estimated at rest and after regadenoson-induced hyperemia. The effect of dosing protocol on dose reduction, PET scanner saturation, relative perfusion, and image quality was compared. The effect of PET saturation on the accuracy of MBF and myocardial flow reserve (MFR) in remote myocardium was assessed with multivariable linear regression.
Results
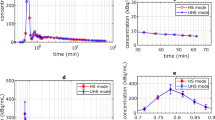
BMI-adjusted dosing was associated with lower administered 82Rb activities (1036.0 ± 274 vs. 1147 ± 274 MBq, p = 0.003) and lower PET scanner saturation incidence (28 vs. 38%, p = 0.006) and severity (median saturation severity index 0.219 ± 0.33 vs. 0.397 ± 0.59%, p = 0.018) compared to weight-adjusted dosing. PET saturation that occurred with either dosing protocol was moderate and resulted in modest remote MBF and MFR biases ranging from 2 to 9% after adjusting for patient age, sex, BMI, rate-pressure product, and LV ejection fraction. No adverse effects of BMI dose adjustment were observed in relative perfusion assessment or image quality.
Conclusions
Patient-specific dosing according to BMI is an effective method for guideline-directed dose reduction while maintaining image quality and accuracy for routine MBF and MFR quantification.





Similar content being viewed by others
Abbreviations
- PET:
-
Positron emission tomography
- MBF:
-
Myocardial blood flow
- MFR:
-
Myocardial flow reserve
- BMI:
-
Body mass index
- TOF:
-
Time of flight
- DTF:
-
Dead time factor
- LV:
-
Left ventricle
References
Murthy VL, Bateman TM, Beanlands RS, et al. Clinical quantification of myocardial blood flow using PET: joint position paper of the SNMMI cardiovascular council and the ASNC. J Nucl Cardiol. 2018;25:269–97.
Sciagrà R, Lubberink M, Hyafil F, et al. EANM procedural guidelines for PET/CT quantitative myocardial perfusion imaging. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-05046-9.
Gould KL, Johnson NP, Bateman TM, et al. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol. 2013;62(18):1639–53.
Schindler TH. Myocardial blood flow: putting it into clinical perspective. J Nucl Cardiol. 2016;23(5):1056–71.
Schindler TH, Dilsizian V. Coronary microvascular dysfunction: clinical considerations and noninvasive diagnosis. JACC Cardiovasc Imaging. 2020;13(1):140–55.
Tio RA, Dabeshlim A, Siebelink H-MJ, et al. Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med. 2009;50(2):214–9.
Herzog BA, Husmann L, Valenta I, et al. Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography: added value of coronary flow reserve. J Am Coll Cardiol. 2009;54(2):150–6.
Ziadi MC, RA DK, Williams KA, et al. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol. 2011;58(7):740–8.
Murthy VL, Naya M, Foster CR, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation. 2011;124(20):2215–24.
Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging. 2013;14(12):1203–10.
Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. N Engl J Med. 2003;349(11):1027–35.
Sciagrà R, Calabretta R, Cipollini F, et al. Myocardial blood flow and left ventricular functional reserve in hypertrophic cardiomyopathy: a 13NH3 gated PET study. Eur J Nucl Med Mol Imaging. 2017;44(5):866–75.
Hajjiri MM, Leavitt MB, Zheng H, Spooner AE, Fischman AJ, Gewirtz H. Comparison of positron emission tomography measurement of adenosine-stimulated absolute myocardial blood flow versus relative myocardial tracer content for physiological assessment of coronary artery stenosis severity and location. J Am Coll Cardiol Img. 2009;2(6):751–8.
Kajander S, Joutsiniemi E, Saraste M, et al. Clinical value of absolute quantification of myocardial perfusion with 15O-water in coronary artery disease. Circ Cardiovasc Imaging. 2011;4(6):678–84.
Ziadi MC, RA dK, Williams K, et al. Does quantification of myocardial flow reserve using rubidium-82 positron emission tomography facilitate detection of multivessel coronary artery disease? J Nucl Cardiol. 2012;19(4):670–80.
Hoff CM, Sørensen J, Christensen NL, Bouchelouche K, Tolbod L. Activity regimes for 82Rb cardiac PET: Effects on absolute MBF and MPI. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-020-02266-2.
Klein R, RA dK. Selection of PET camera and implications on the reliability and accuracy of absolute myocardial blood flow quantification. Curr Cardiol Rep. 2020;22(10).
Moody JB, Lee BC, Corbett JR, Ficaro EP, Murthy VL. Precision and accuracy of clinical quantification of myocardial blood flow by dynamic PET: a technical perspective. J Nucl Cardiol. 2015;22(5):935–51.
Cerqueira MD, Allman KC, Ficaro EP, et al. Recommendations for reducing radiation exposure in myocardial perfusion imaging. J Nucl Cardiol. 2010;17(4):709–18.
Fazel R, Gerber TC, Balter S, et al. Approaches to enhancing radiation safety in cardiovascular imaging. A scientific statement from the American Heart Association. Circulation. 2014;130(19):1730–48.
Case JA, RA dK, Slomka PJ, smith MF, Heller GV, Cerqueira MD. Status of cardiovascular PET radiation exposure and strategies for reduction: an information statement from the cardiovascular PET task force. J Nucl Cardiol. 2017;24(4):1427–39.
Tout D, Tonge C, Muthu S, Arumugam P. Assessment of a protocol for routine simultaneous myocardial blood flow measurement and standard myocardial perfusion imaging with rubidium-82 on a high count rate positron emission tomography system. Nucl Med Commun. 2012;33(11):1202–11.
Lassen ML, Manabe O, Otaki Y, et al. 3D PET/CT 82Rb PET myocardial blood flow quantification: comparison of half-dose and full-dose protocols. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04811-0.
van Dijk JD, Dotinga M, Jager PL, et al. Body weight-dependent Rubidium-82 activity results in constant image quality in myocardial perfusion imaging with PET. J Nucl Cardiol. 2020. https://doi.org/10.1007/s12350-019-01875-w.
Esteves FP, Nye JA, Khan A, et al. Prompt-gamma compensation in Rb-82 myocardial perfusion 3D PET/CT. J Nucl Cardiol. 2010;17(2):247–53.
Armstrong IS, Memmott MJ, Tonge CM, Arumugam P. The impact of prompt gamma compensation on myocardial blood flow measurements with rubidium-82 dynamic PET. J Nucl Cardiol. 2016:1–10.
Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: the Michigan method for quantitative nuclear cardiology. J Nucl Cardiol. 2007;14(4):455–65.
Renaud JM, Yip K, Guimond J, et al. Characterization of 3D PET systems for accurate quantification of myocardial blood flow. J Nucl Med. 2017;58(1):103–9.
Moody JB, Murthy VL, Lee BC, Corbett JR, Ficaro EP. Variance estimation for myocardial blood flow by dynamic PET. IEEE Trans Med Imaging. 2015;34(11):2343–53.
Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the American Heart Association. Circulation. 2002;105(4):539–42.
Slomka PJ, Nishina H, Berman DS, et al. Automated quantification of myocardial perfusion SPECT using simplified normal limits. J Nucl Cardiol. 2005;12(1):66–77.
Velilla S. A note on the multivariate box—cox transformation to normality. Stat Probab Lett. 1993;17(4):259–63.
Fox J, Weisberg S. An R companion to applied regression. Third edition. Thousand Oaks, California: SAGE Publications, Inc; 2019.
R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for statistical Computing. 2012; http://www.R-project.org/.
Fox J, Weisberg S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J Stat Softw. 2018;87(1):1–27.
Virtanen P, Gommers R, Oliphant TE, et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. 2020;17(3):261–72.
Klein R, Ocneanu A, Renaud JM, Ziadi MC, Beanlands RSB, RA DK. Consistent tracer administration profile improves test–retest repeatability of myocardial blood flow quantification with 82Rb dynamic PET imaging. J Nucl Cardiol. 2016:1–13.
Kitkungvan D, Johnson NP, Roby AE, Patel MB, Kirkeeide R, Gould KL. Routine clinical quantitative rest stress myocardial perfusion for managing coronary artery disease: clinical relevance of test-retest variability. JACC Cardiovasc Imaging. 2017;10(5):565–77.
van Dijk JD, Jager PL, van Osch JAC, Khodaverdi M, van Dalen JA. Comparison of maximal rubidium-82 activities for myocardial blood flow quantification between digital and conventional PET systems. J Nucl Cardiol. 2018. https://doi.org/10.1007/s12350-017-1156-9.
Case JA, Courter SA, Moloney E, Bateman TM. Evaluation of image quality, radiation dose and quantification of myocardial blood using a weight-based, constant activity Rb-82 infusion system In: Abstracts of Original Contributions ASNC2020. Springer. 2020.
Casey M, Gadagkar H, Newport D. A component based method for normalization in volume PET. In: Grangeat P, Amans J, editors. Aix-les-Bains. Savoie, France: Kluwer Academic; 1995. p. 67–71. Available at: www.fully3d.org/1995/3D95Proceedings.pdf.
Acknowledgements
The authors thank Michael E. Casey, PhD, and Mary Germino, PhD, for helpful discussions on PET saturation and dead time factor calculations.
Funding
VLM is supported by grants R01AG059729 from the National Institute on Aging, U01DK123013 from the National Institute of Diabetes and Digestive and Kidney Disease, and R01HL136685 from the National Heart, Lung, and Blood Institute as well as the Melvyn Rubenfire Professorship in Preventive Cardiology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
This article does not contain any studies with human participants performed by any of the authors.
Consent to participate
All data were anonymized before analysis, and informed consent was not required under an exemption from the University of Michigan Institutional Review Board.
Conflict of interest
LAM declares that she has no conflict of interest; JBM, JMR, AP, and TH are employees of INVIA; AMS is an employee of Siemens Healthineers; EPF is a stockholder in INVIA. VLM has received research grants and speaking honoraria from Siemens Medical Imaging and serves as a scientific advisor for Ionetix and owns stock options in the same. He owns stock in GE and Cardinal Health, has received expert witness payments on behalf of Jubilant DraxImage and a speaking honorarium from 2Quart Medical, and receives nonfinancial research support from INVIA.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Cardiology
Appendix
Dead time correction for our PET-CT scanner (Siemens Biograph mCT) was performed as part of the vendor’s component-based normalization [41]. The average total dead time factor was estimated as a function of detector singles rate using the following formula:
Supplementary information
ESM 1
(PDF 1.47 mb)
Rights and permissions
About this article
Cite this article
Arida-Moody, L., Moody, J.B., Renaud, J.M. et al. Effects of two patient-specific dosing protocols on measurement of myocardial blood flow with 3D 82Rb cardiac PET. Eur J Nucl Med Mol Imaging 48, 3835–3846 (2021). https://doi.org/10.1007/s00259-021-05385-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05385-1