Abstract
Purpose
[68Ga]Ga-labeled fibroblast activation protein inhibitors ([68Ga]Ga-FAPi) have shown promising preclinical and clinical results in PET imaging. The present study aimed to evaluate the biodistribution, pharmacokinetics, and dosimetry of [68Ga]Ga-DOTA.SA.FAPi, another modified FAPi tracer, and performed a head-to-head comparison with [18F]F-FDG PET/CT scans in patients with various cancers.
Methods
In this prospective study, patients underwent both [18F]F-FDG and [68Ga]Ga-DOTA.SA.FAPi PET/CT scans 60 min post-injection (p.i.). Dosimetry studies were conducted in three patients using [68Ga]Ga-DOTA.SA.FAPi serial time-point imaging. The absorbed dose was calculated using OLINDA/EXM 2.2 software. Quantification of the uptake of the tracers was assessed using standardized uptake values corrected for lean body mass (SUL).
Results
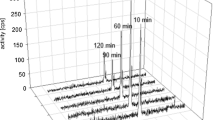
Fifty-four patients (mean age; 48.4 years) with 14 types of cancers involving 37% breast, 24% lung, 7.4% head and neck (H&N), and remaining 31.6% patients with other histologies were evaluated prospectively. Physiological uptake of [68Ga]Ga-DOTA.SA.FAPi was observed in the liver, kidneys, pancreas, heart contents, and to a lesser extent in the lacrimals, oral mucosa, salivary glands, and thyroid glands. Uptake in the target lesions on [68Ga]Ga-DOTA.SA.FAPi scan was initiated at 10 min, and no additional lesions were detected in the delayed acquisition time points. The pancreas was the organ with the highest absorbed dose (5.46E-02 mSv/MBq). While the patient-based comparison between the radiotracers revealed complete concordance in the detection of primary, pleural thickening, bone and liver metastases, and second primary malignancy, discordant findings were observed in the detection of lymph node (7.5%), lung nodules (5.6%), and brain metastases (2%). According to the site of primary disease, patients with H&N cancers demonstrated the highest SULpeak and average (avg) values on [68Ga]Ga-DOTA.SA-FAPi which was similar to the values of [18F]F-FDG [(SULpeak: 15.4 vs. 14.2; P-0.680) (SULavg: 8.3 vs. 7.9; P-0.783)]. The lowest uptake was observed in lung cancers with both the radiotracers [(SULpeak: 5.8 vs. 7.4; P-0.238) (SULavg: 4.9 vs. 5.3; P-0.313)]. A significantly higher SULpeak and SULavg for brain metastases to normal brain parenchyma ratios were observed on [68Ga]Ga-DOTA.SA.FAPi in contrast to the [18F]F-FDG values {SULpeak: median: 59.3 (IQR: 33.5–130.8) versus 1.5 (1–2.3); P-0.028}. Except for brain metastases, comparable SULpeak and average values were noted between the radiotracers in all other regions of metastases with no significant difference.
Conclusion
[68Ga]Ga-DOTA.SA.FAPi is a promising alternative among the FAPI class of molecules and performed well as compared to standard-of-care radiotracer [18F]F-FDG in the diagnosis of various cancers.








Similar content being viewed by others
Data availability
The data and material are available.
References
Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8:519–30.
Mankoff DA, Eary JF, Link JM, Muzi M, Rajendran JG, Spence AM, et al. Tumor-specific positron emission tomography imaging in patients: [18F] fluorodeoxyglucose and beyond. Clin Cancer Res. 2007;13:3460–9.
Chang JM, Lee HJ, Goo JM, Lee HY, Lee JJ, Chung JK, et al. False positive and false negative FDG-PET scans in various thoracic diseases. Korean J Radiol. 2006;7:57–69.
Davidson B, Trope CG, Reich R. The role of the tumor stroma in ovarian cancer. Front Oncol. 2014;4:104.
Garin-Chesa P, Old LJ, Rettig WJ. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc Natl Acad Sci. 1990;87:7235–9.
Cheng DJ, Dunbrack RL, Valianou M, Rogatko A, Alpaugh RK, Weine LM. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62:4767–72.
Liu R, Li H, Liu L, Yu J, Ren X. Fibroblast activation protein: a potential therapeutic target in cancer. Cancer Biol Ther. 2012;13:123–9.
Jansen K, Heirbaut L, Verkerk R, Cheng JD, Joossens J, Cos P, et al. Extended structure-activity relationship and pharmacokinetic investigation of (4-quinolinoyl)glycyl-2-cyanopyrrolidine inhibitors of fibroblast activation protein (FAP). J Med Chem. 2014;57:3053–74.
Giesel FL, Kratochwil C, Lindner T, Marschalek MM, Loktev A, Lehnert W, et al. 68Ga-FAPI PET/CT: biodistribution and preliminary dosimetry estimate of 2 DOTA-containing FAP-targeting agents in patients with various cancers. J Nucl Med. 2019;60:386–92.
Kratochwil C, Flechsig P, Lindner T, Abderrahim L, Altmann A, Mier W, et al. 68Ga-FAPI PET/CT: tracer uptake in 28 different kinds of cancer. J Nucl Med. 2019;60:801–5.
Meyer C, Dahlbom M, Lindner T, Vauclin S, Mona C, Slavik R, et al. Radiation dosimetry and biodistribution of 68Ga-FAPI-46 PET imaging in cancer patients. J Nucl Med. 2020;61:1171-7.
Loktev A, Lindner T, Burger EM, Altmann A, Giesel F, Kratochwil C, et al. Development of fibroblast activation protein-targeted radiotracers with improved tumor retention. J Nucl Med. 2019;60:1421–9.
Moon ES, Elvas F, Vliegen G, Lombaerde SD, Vangestel C, Bruycker SD, et al. Targeting fibroblast activation protein (FAP): next generation PET radiotracers using squaramide coupled bifunctional DOTA and DATA 5m chelators. EJNMMI Radiopharm Chem. 2020;5:19. https://doi.org/10.1186/s41181-020-00102-z.
Ballal S, Yadav MP, Kramer V, Moon ES, Roesch F, Tripathi M, et al. A theranostic approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-guided [177Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: new frontier in targeted radionuclide therapy. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04990-w.
Valentin J. Basic anatomical and physiological data for use in radiological protection: reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann ICRP. 2002;32:1–277.
Ferrer L, Kraeber-Bodere F, Bodet-Milin C, Rousseau C, Gouill SL, Wegener WA, et al. Three methods assessing redmarrow dosimetry in lymphoma patients treated with radioimmunotherapy. Cancer. 2010;116:1093–100.
Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: evolving considerations for PET response criteria in solid tumors. J Nucl Med. 2009;50(Suppl 1):122–50.
Mandrekar JN. Measures of interrater agreement. J Thorac Oncol. 2011;6(1):6–7.
Busek P, Hrabal P, Fric P, Sedo A. Co-expression of the homologous proteases fibroblast activation protein and dipeptidyl peptidase-IV in the adult human Langerhans islets. Histochem Cell Biol. 2015;143:497–504.
Chen H, Pang Y, Wu J, Zhao L, Hao B, Wu J, et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur J Nucl Med Mol Imaging. 2020;47:1820–32.
Liao Y, Ni Y, He R, Liu W, Du J. Clinical implications of fibroblast activation protein-α in non-small cell lung cancer after curative resection: a new predictor for prognosis. J Cancer Res Clin Oncol. 2013;139:1523–8.
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y, et al. Downregulation of FAP suppresses cell proliferation and metastasis through PTEN/PI3K/AKT and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death Dis. 2014;5:e1155.
Zhang Y, Tang H, Cai J, Zhang T, Guo J, Feng D, et al. Ovarian cancer-associated fibroblasts contribute to epithelial ovarian carcinoma metastasis by promoting angiogenesis, lymphangiogenesis and tumor cell invasion. Cancer Lett. 2011;303:47–55.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical clearance
Ref. No IECPG-22/2020.
Informed consent
we obtained written informed consent from all the patients before commencing the investigational PET/CT Scan.
Disclaimer
The current work has not been submitted for review or is not under acceptance for publication in any journal.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Radiopharmacy
Supplementary information
ESM 1
(DOCX 25 kb).
Rights and permissions
About this article
Cite this article
Ballal, S., Yadav, M.P., Moon, E.S. et al. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur J Nucl Med Mol Imaging 48, 1915–1931 (2021). https://doi.org/10.1007/s00259-020-05132-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05132-y