Abstract
Purpose
In vivo measurement of the spatial distribution of neurofibrillary tangle pathology is critical for early diagnosis and disease monitoring of Alzheimer’s disease (AD).
Methods
Forty-nine participants were scanned with 18F-PI-2620 PET to examine the distribution of this novel PET ligand throughout the course of AD: 36 older healthy controls (HC) (age range 61 to 86), 11 beta-amyloid+ (Aβ+) participants with cognitive impairment (CI; clinical diagnosis of either mild cognitive impairment or AD dementia, age range 57 to 86), and 2 participants with semantic variant primary progressive aphasia (svPPA, age 66 and 78). Group differences in brain regions relevant in AD (medial temporal lobe, posterior cingulate cortex, and lateral parietal cortex) were examined using standardized uptake value ratios (SUVRs) normalized to the inferior gray matter of the cerebellum.
Results
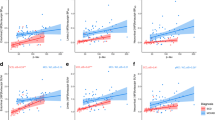
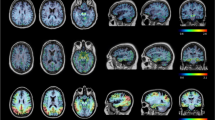
SUVRs in target regions were relatively stable 60 to 90 min post-injection, with the exception of very high binders who continued to show increases over time. Robust elevations in 18F-PI-2620 were observed between HC and Aβ+ CI across all AD regions. Within the HC group, older age was associated with subtle elevations in target regions. Mildly elevated focal uptake was observed in the anterior temporal pole in one svPPA patient.
Conclusion
Preliminary results suggest strong differences in the medial temporal lobe and cortical regions known to be impacted in AD using 18F-PI-2620 in patients along the AD trajectory. This work confirms that 18F-PI-2620 holds promise as a tool to visualize tau aggregations in AD.








Similar content being viewed by others
References
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 2012;123:1–11. https://doi.org/10.1007/s00401-011-0910-3.
Chien DT, Bahri S, Szardenings AK, Walsh JC, Mu F, Su MY, et al. Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J Alzheimers Dis. 2013;34:457–68. https://doi.org/10.3233/JAD-122059.
Fleisher AS, Pontecorvo MJ, Devous MD Sr, Lu M, Arora AK, Truocchio SP, et al. Positron emission tomography imaging with [18F]flortaucipir and postmortem assessment of Alzheimer disease neuropathologic changes. JAMA Neurol. 2020. https://doi.org/10.1001/jamaneurol.2020.0528.
Johnson KA, Schultz A, Betensky RA, Becker JA, Sepulcre J, Rentz D, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol. 2016;79:110–9. https://doi.org/10.1002/ana.24546.
Pontecorvo MJ, Devous MD Sr, Navitsky M, Lu M, Salloway S, Schaerf FW, et al. Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain. 2017;140:748–63. https://doi.org/10.1093/brain/aww334.
Sperling RA, Mormino EC, Schultz AP, Betensky RA, Papp KV, Amariglio RE, et al. The impact of amyloid-beta and tau on prospective cognitive decline in older individuals. Ann Neurol. 2019;85:181–93. https://doi.org/10.1002/ana.25395.
Jack CR Jr, Wiste HJ, Therneau TM, Weigand SD, Knopman DS, Mielke MM, et al. Associations of amyloid, tau, and neurodegeneration biomarker profiles with rates of memory decline among individuals without dementia. JAMA. 2019;321:2316–25. https://doi.org/10.1001/jama.2019.7437.
Scholl M, Lockhart SN, Schonhaut DR, O’Neil JP, Janabi M, Ossenkoppele R, et al. PET imaging of tau deposition in the aging human brain. Neuron. 2016;89:971–82. https://doi.org/10.1016/j.neuron.2016.01.028.
Ossenkoppele R, Schonhaut DR, Scholl M, Lockhart SN, Ayakta N, Baker SL, et al. Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer’s disease. Brain. 2016;139:1551–67. https://doi.org/10.1093/brain/aww027.
Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer disease: a complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. https://doi.org/10.1097/NEN.0b013e3181919a48.
Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004;61:378–84. https://doi.org/10.1001/archneur.61.3.378.
Bullich S, Barret O, Constantinescu C, Sandiego C, Mueller A, Berndt M, et al. Evaluation of dosimetry, quantitative methods and test-retest variability of (18)F-PI-2620 PET for the assessment of tau deposits in the human brain. J Nucl Med. 2019. https://doi.org/10.2967/jnumed.119.236240.
Kroth H, Oden F, Molette J, Schieferstein H, Capotosti F, Mueller A, et al. Discovery and preclinical characterization of [(18)F]PI-2620, a next-generation tau PET tracer for the assessment of tau pathology in Alzheimer’s disease and other tauopathies. Eur J Nucl Med Mol Imaging. 2019;46:2178–89. https://doi.org/10.1007/s00259-019-04397-2.
Beyer L, Nitschmann A, Barthel H, van Eimeren T, Unterrainer M, Sauerbeck J, et al. Early-phase [(18)F]PI-2620 tau-PET imaging as a surrogate marker of neuronal injury. Eur J Nucl Med Mol Imaging. 2020. https://doi.org/10.1007/s00259-020-04788-w.
Mueller A, Bullich S, Barret O, Madonia J, Berndt M, Papin C, et al. Tau PET imaging with (18)F-PI-2620 in patients with Alzheimer’s disease and healthy controls: a first-in-human study. J Nucl Med. 2019. https://doi.org/10.2967/jnumed.119.236224.
Sousa JM, Appel L, Engstrom M, Papadimitriou S, Nyholm D, Larsson EM, et al. Evaluation of zero-echo-time attenuation correction for integrated PET/MR brain imaging-comparison to head atlas and (68)Ge-transmission-based attenuation correction. EJNMMI Phys. 2018;5:20. https://doi.org/10.1186/s40658-018-0220-0.
Vemuri P, Lowe VJ, Knopman DS, Senjem ML, Kemp BJ, Schwarz CG, et al. Tau-PET uptake: regional variation in average SUVR and impact of amyloid deposition. Alzheimers Dement (Amst). 2017;6:21–30. https://doi.org/10.1016/j.dadm.2016.12.010.
Grabner G, Janke AL, Budge MM, Smith D, Pruessner J, Collins DL. Symmetric atlasing and model based segmentation: an application to the hippocampus in older adults. Med Image Comput Comput Assist Interv. 2006;9:58–66. https://doi.org/10.1007/11866763_8.
Fischl B. FreeSurfer Neuroimage. 2012;62:774–81. https://doi.org/10.1016/j.neuroimage.2012.01.021.
Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. https://doi.org/10.1016/s0896-6273(02)00569-x.
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–80. https://doi.org/10.1016/j.neuroimage.2006.01.021.
Tang-Wai DF, Graff-Radford NR, Boeve BF, Dickson DW, Parisi JE, Crook R, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63:1168–74. https://doi.org/10.1212/01.wnl.0000140289.18472.15.
Jack CR Jr, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA research framework: toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–62. https://doi.org/10.1016/j.jalz.2018.02.018.
Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh compound-B. Ann Neurol. 2004;55:306–19. https://doi.org/10.1002/ana.20009.
Scholl M, Maass A, Mattsson N, Ashton NJ, Blennow K, Zetterberg H, et al. Biomarkers for tau pathology. Mol Cell Neurosci. 2019;97:18–33. https://doi.org/10.1016/j.mcn.2018.12.001.
Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N Engl J Med. 2019;380:1408–20. https://doi.org/10.1056/NEJMoa1812840.
Ostrowitzki S, Lasser RA, Dorflinger E, Scheltens P, Barkhof F, Nikolcheva T, et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimers Res Ther. 2017;9:95. https://doi.org/10.1186/s13195-017-0318-y.
Giacobini E, Gold G. Alzheimer disease therapy--moving from amyloid-beta to tau. Nat Rev Neurol. 2013;9:677–86. https://doi.org/10.1038/nrneurol.2013.223.
Teng E, Ward M, Manser PT, Sanabria-Bohorquez S, Ray RD, Wildsmith KR, et al. Cross-sectional associations between [(18)F]GTP1 tau PET and cognition in Alzheimer’s disease. Neurobiol Aging. 2019;81:138–45. https://doi.org/10.1016/j.neurobiolaging.2019.05.026.
Kuwabara H, Comley RA, Borroni E, Honer M, Kitmiller K, Roberts J, et al. Evaluation of (18)F-RO-948 PET for quantitative assessment of tau accumulation in the human brain. J Nucl Med. 2018;59:1877–84. https://doi.org/10.2967/jnumed.118.214437.
Betthauser TJ, Cody KA, Zammit MD, Murali D, Converse AK, Barnhart TE, et al. In vivo characterization and quantification of neurofibrillary tau PET radioligand (18)F-MK-6240 in humans from Alzheimer disease dementia to young controls. J Nucl Med. 2019;60:93–9. https://doi.org/10.2967/jnumed.118.209650.
Scholl M, Ossenkoppele R, Strandberg O, Palmqvist S, Swedish Bio FS, Jogi J, et al. Distinct 18F-AV-1451 tau PET retention patterns in early- and late-onset Alzheimer’s disease. Brain. 2017;140:2286–94. https://doi.org/10.1093/brain/awx171.
Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59.
Jack CR Jr, Wiste HJ, Schwarz CG, Lowe VJ, Senjem ML, Vemuri P, et al. Longitudinal tau PET in ageing and Alzheimer’s disease. Brain. 2018;141:1517–28. https://doi.org/10.1093/brain/awy059.
Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K, et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38:530–43. https://doi.org/10.1523/JNEUROSCI.2028-17.2017.
Lowe VJ, Curran G, Fang P, Liesinger AM, Josephs KA, Parisi JE, et al. An autoradiographic evaluation of AV-1451 tau PET in dementia. Acta Neuropathol Commun. 2016;4:58. https://doi.org/10.1186/s40478-016-0315-6.
Lockhart SN, Ayakta N, Winer JR, La Joie R, Rabinovici GD, Jagust WJ. Elevated 18F-AV-1451 PET tracer uptake detected in incidental imaging findings. Neurology. 2017;88:1095–7. https://doi.org/10.1212/WNL.0000000000003724.
Pierce AL, Kawas CH. Dementia in the oldest old: beyond Alzheimer disease. PLoS Med. 2017;14:e1002263. https://doi.org/10.1371/journal.pmed.1002263.
Bergeron D, Gorno-Tempini ML, Rabinovici GD, Santos-Santos MA, Seeley W, Miller BL, et al. Prevalence of amyloid-beta pathology in distinct variants of primary progressive aphasia. Ann Neurol. 2018;84:729–40. https://doi.org/10.1002/ana.25333.
Smith R, Santillo AF, Waldo ML, Strandberg O, Berron D, Vestberg S, et al. (18)F-Flortaucipir in TDP-43 associated frontotemporal dementia. Sci Rep. 2019;9:6082. https://doi.org/10.1038/s41598-019-42625-9.
Schaeverbeke J, Celen S, Cornelis J, Ronisz A, Serdons K, Van Laere K, et al. Binding of [(18)F]AV1451 in post mortem brain slices of semantic variant primary progressive aphasia patients. Eur J Nucl Med Mol Imaging. 2019. https://doi.org/10.1007/s00259-019-04631-x.
Acknowledgments
The authors acknowledge Life Molecular Imaging for providing the precursor for this study.
Funding
This study was funded by the NIH (K01-AG051718 and R21-AG058859, Mormino; R01-AG048076, Wagner; P50-AG047366, Henderson; R01-AG061120, Zeineh), General Electric (GE), the Stanford Wu Tsai Neuroscience Institute, and the Stanford Precision Health and Integrated Diagnostics Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Author Elizabeth Mormino receives research funds from the National Institutes of Health grants K01-AG051718 and R21-AG058859. Dr. Mormino has been a consultant for Eli Lilly, Biogen Idec, F Hoffmann-La Roche Ltd., Janssen, and Alector.
Author Michael Zeineh receives research funds from the National Institutes of Health grant R01-AG061120 and from General Electric (GE).
Michelle James reports travel compensation from World Molecular Imaging Congress (WMIC) to present on Emerging neuroimaging techniques and present at an educational session on “best practices for small animal PET imaging.”
Author Sharon Sha receives research support from Biogen Idec, F Hoffmann-La Roche Ltd., Genentech, and Novartis for her role as Investigator in Clinical Trials. Dr. Sha has been a consultant for Abelson Taylor, Baird, Clearview Healthcare Partners, SelfCare Catalysts Inc., and the University of Southern California. Dr. Sha is funded by National Institutes of Health grants P50 AG047366 (Stanford Alzheimer’s Disease Center) and R01 AG048076.
Author Greg Zaharchuk has received research support from GE Healthcare, LifeMI, Stanford AI Laboratory (SAIL), and the National Institutes of Health grants P50 AG047366. Dr. Zaharchuk has equity in Subtle Medical.
Author Anthony Wagner receives research funds from the National Institutes of Health grant R01-AG048076.
All other authors declare that he/she has no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOCX 957 kb)
Rights and permissions
About this article
Cite this article
Mormino, E.C., Toueg, T.N., Azevedo, C. et al. Tau PET imaging with 18F-PI-2620 in aging and neurodegenerative diseases. Eur J Nucl Med Mol Imaging 48, 2233–2244 (2021). https://doi.org/10.1007/s00259-020-04923-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04923-7