Abstract
Purpose
The widespread use of gallium-68-labelled somatostatin analogue (SSA) PET, the current standard for somatostatin receptor (SSTR) imaging, is limited by practical and economic challenges that could be overcome by a fluorine-18-labelled alternative, such as the recently introduced [18F]AlF-NOTA-octreotide ([18F]AlF-OC). This prospective trial aimed to evaluate safety, dosimetry, biodistribution, pharmacokinetics and lesion targeting of [18F]AlF-OC and perform the first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour (NET) patients.
Methods
Six healthy volunteers and six NET patients with a previous clinical [68Ga]Ga-DOTATATE PET were injected with an IV bolus of 4 MBq/kg [18F]AlF-OC. Healthy volunteers underwent serial whole-body PET scans from time of tracer injection up to 90 min post-injection, with an additional PET/CT at 150 and 300 min post-injection. In patients, a 45-min dynamic PET was acquired and three whole-body PET scans at 60, 90 and 180 min post-injection. Absorbed organ doses and effective doses were calculated using OLINDA/EXM. Normal organ uptake (SUVmean) and tumour lesion uptake (SUVmax and tumour-to-background ratio (TBR)) were measured. A lesion-by-lesion analysis was performed and the detection ratio (DR), defined as the fraction of detected lesions was determined for each tracer.
Results
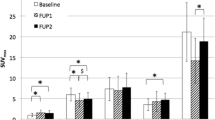
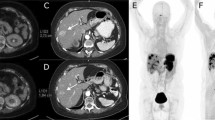
[18F]AlF-OC administration was safe and well tolerated. The highest dose was received by the spleen (0.159 ± 0.062 mGy/MBq), followed by the urinary bladder wall (0.135 ± 0.046 mGy/mBq) and the kidneys (0.070 ± 0.018 mGy/MBq), in accordance with the expected SSTR-specific uptake in the spleen and renal excretion of the tracer. The effective dose was 22.4 ± 4.4 μSv/MBq. The physiologic uptake pattern of [18F]AlF-OC was comparable to [68Ga]Ga-DOTATATE. Mean tumour SUVmax was lower for [18F]AlF-OC (12.3 ± 6.5 at 2 h post-injection vs. 18.3 ± 9.5; p = 0.03). However, no significant differences were found in TBR (9.8 ± 6.7 at 2 h post-injection vs. 13.6 ± 11.8; p = 0.35). DR was high and comparable for both tracers (86.0% for [68Ga]Ga-DOTATATE vs. 90.1% for [18F]AlF-OC at 2 h post-injection; p = 0.68).
Conclusion
[18F]AlF-OC shows favourable kinetic and imaging characteristics in patients that warrant further head-to-head comparison to validate [18F]AlF-OC as a fluorine-18-labelled alternative for gallium-68-labelled SSA clinical PET.
Trial registration
Clinicaltrials.gov: NCT03883776, EudraCT: 2018-002827-40






Similar content being viewed by others
References
Bozkurt MF, Virgolini I, Balogova S, Beheshti M, Rubello D, Decristoforo C, et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur J Nucl Med Mol Imaging. 2017;44:1588–601.
Smit Duijzentkunst DA, Kwekkeboom DJ, Bodei L. Somatostatin receptor 2-targeting compounds. J Nucl Med. 2017;58:54S–60S.
Pauwels E, Cleeren F, Bormans G, Deroose CM. Somatostatin receptor PET ligands - the next generation for clinical practice. Am J Nucl Med Mol Imaging. 2018;8:311–31.
Sadowski SM, Neychev V, Millo C, Shih J, Nilubol N, Herscovitch P, et al. Prospective study of 68Ga-DOTATATE positron emission tomography/computed tomography for detecting gastro-entero-pancreatic neuroendocrine tumors and unknown primary sites. J Clin Oncol. 2016;34:588–96.
Gabriel M, Decristoforo C, Kendler D, Dobrozemsky G, Heute D, Uprimny C, et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med. 2007;48:508–18.
Deppen SA, Liu E, Blume JD, Clanton J, Shi C, Jones-Jackson LB, et al. Safety and efficacy of 68Ga-DOTATATE PET/CT for diagnosis, staging, and treatment management of neuroendocrine tumors. J Nucl Med. 2016;57:708–14.
Van Binnebeek S, Vanbilloen B, Baete K, Terwinghe C, Koole M, Mottaghy FM, et al. Comparison of diagnostic accuracy of 111In-pentetreotide SPECT and 68Ga-DOTATOC PET/CT: a lesion-by-lesion analysis in patients with metastatic neuroendocrine tumours. Eur Radiol. 2016;26:900–9.
Hope TA, Bergsland EK, Bozkurt MF, Graham M, Heaney AP, Herrmann K, et al. Appropriate use criteria for somatostatin receptor PET imaging in neuroendocrine tumors. J Nucl Med. 2018;59:66–74.
Dubash SR, Keat N, Mapelli P, Twyman F, Carroll L, Kozlowski K, et al. Clinical translation of a click-labeled 18F-octreotate radioligand for imaging neuroendocrine tumors. J Nucl Med. 2016;57:1207–13.
Long T, Yang N, Zhou M, Chen D, Li Y, Li J, et al. Clinical application of 18F-AlF-NOTA-Octreotide PET/CT in combination with 18F-FDG PET/CT for imaging neuroendocrine neoplasms. Clin Nucl Med. 2019;44:452–8.
Pauwels E, Cleeren F, Tshibangu T, Koole M, Serdons K, Dekervel J, et al. Al18F-NOTA-octreotide: first comparison with 68Ga-DOTATATE in a neuroendocrine tumour patient. Eur J Nucl Med Mol Imaging. 2019;46:2398–9.
Ilhan H, Lindner S, Todica A, Cyran CC, Tiling R, Auernhammer CJ, et al. Biodistribution and first clinical results of 18F-SiFAlin-TATE PET: a novel 18F-labeled somatostatin analog for imaging of neuroendocrine tumors. Eur J Nucl Med Mol Imaging. 2020;47:870–80.
Laverman P, McBride WJ, Sharkey RM, Eek A, Joosten L, Oyen WJ, et al. A novel facile method of labeling octreotide with 18F-fluorine. J Nucl Med. 2010;51:454–61.
Tshibangu T, Cawthorne C, Serdons K, Pauwels E, Gsell W, Bormans G, et al. Automated GMP compliant production of [18F]AlF-NOTA-octreotide. EJNMMI Radiopharm Chem. 2020;5:4.
International Commission on Radiological Protection. Limits for intakes of radionuclides by workers. ICRP publication 30 (Part 1). Ann ICRP. 1979;2.
Van Binnebeek S, Koole M, Terwinghe C, Baete K, Vanbilloen B, Haustermans K, et al. Dynamic 68Ga-DOTATOC PET/CT and static image in NET patients. Correlation of parameters during PRRT. Nuklearmedizin. 2016;55:104–14.
Ilan E, Velikyan I, Sandstrom M, Sundin A, Lubberink M. Tumor-to-blood ratio for assessment of somatostatin receptor density in neuroendocrine tumors using 68Ga-DOTATOC and 68Ga-DOTATATE. J Nucl Med. 2020;61:217–21.
Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1985;5:584–90.
Meisetschlaeger G, Poethko T, Stahl A, Wolf I, Scheidhauer K, Schottelius M, et al. Gluc-Lys([18F]FP)-TOCA PET in patients with SSTR-positive tumors: biodistribution and diagnostic evaluation compared with [111In]DTPA-octreotide. J Nucl Med. 2006;47:566–73.
Laverman P, D'Souza CA, Eek A, McBride WJ, Sharkey RM, Oyen WJ, et al. Optimized labeling of NOTA-conjugated octreotide with F-18. Tumor Biol. 2012;33:427–34.
Shastry M, Kayani I, Wild D, Caplin M, Visvikis D, Gacinovic S, et al. Distribution pattern of 68Ga-DOTATATE in disease-free patients. Nucl Med Commun. 2010;31:1025–32.
Haug AR, Rominger A, Mustafa M, Auernhammer C, Goke B, Schmidt GP, et al. Treatment with octreotide does not reduce tumor uptake of 68Ga-DOTATATE as measured by PET/CT in patients with neuroendocrine tumors. J Nucl Med. 2011;52:1679–83.
Cherk MH, Kong G, Hicks RJ, Hofman MS. Changes in biodistribution on 68Ga-DOTA-Octreotate PET/CT after long acting somatostatin analogue therapy in neuroendocrine tumour patients may result in pseudoprogression. Cancer Imaging. 2018;18:3.
Sandstrom M, Velikyan I, Garske-Roman U, Sorensen J, Eriksson B, Granberg D, et al. Comparative biodistribution and radiation dosimetry of 68Ga-DOTATOC and 68Ga-DOTATATE in patients with neuroendocrine tumors. J Nucl Med. 2013;54:1755–9.
Velikyan I, Sundin A, Eriksson B, Lundqvist H, Sorensen J, Bergstrom M, et al. In vivo binding of [68Ga]-DOTATOC to somatostatin receptors in neuroendocrine tumours--impact of peptide mass. Nucl Med Biol. 2010;37:265–75.
Koumarianou E, Pawlak D, Korsak A, Mikolajczak R. Comparison of receptor affinity of natSc-DOTA-TATE versus natGa-DOTA-TATE. Nucl Med Rev Cent East Eur. 2011;14:85–9.
Acknowledgements
The authors explicitly want to thank Mr. Kwinten Porters and Mr. Jef Van Loock for their contributions to the scanning and data handling, and the PET radiopharmacy team and medical physics team of UZ Leuven for their skilled contributions.
Funding
This research was funded by the project from “Kom op tegen Kanker”: “PET/MR imaging of the norepinephrine transporter and somatostatin receptor in neural crest and neuroendocrine tumours for better radionuclide therapy selection” and received support from Research Foundation – Flanders (FWO) (G0D8817N). Frederik Cleeren is a Postdoctoral Fellow of FWO (12R3119N). Christophe M. Deroose is a Senior Clinical Investigator at FWO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Eric Van Cutsem has received research grants and personal fees for consultancy from Amgen, Bayer, Boehringer Ingelheim, Celgene, Ipsen, Lilly, Roche, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche and Servier. Chris Verslype has received research grants and performed consultancy services for Novartis and Ipsen. Koen Van Laere has performed consultancy services and contract research through KU Leuven for GE Healthcare, Merck, Janssen Pharmaceuticals, UCB, Syndesi Therapeutics, Curasen, Celgene and Eikonizo. Guy Bormans has performed funded contract research through KU Leuven with Eikonizo, Merck, Celgene, Janssen Pharmaceuticals, Lundbeck and UCB. Christophe M. Deroose has been a consultant through KU Leuven for Novartis, Terumo, AAA, Ipsen, Sirtex, Bayer outside the scope of the submitted work. There are no other conflicts of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (Ethische Commissie Onderzoek UZ/KU Leuven S61727) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Oncology - General
Rights and permissions
About this article
Cite this article
Pauwels, E., Cleeren, F., Tshibangu, T. et al. [18F]AlF-NOTA-octreotide PET imaging: biodistribution, dosimetry and first comparison with [68Ga]Ga-DOTATATE in neuroendocrine tumour patients. Eur J Nucl Med Mol Imaging 47, 3033–3046 (2020). https://doi.org/10.1007/s00259-020-04918-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04918-4