Abstract
Purpose
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disorder with on average a 1-year delay between symptom onset and diagnosis. Studies have demonstrated the value of [18F]-FDG PET as a sensitive diagnostic biomarker, but the discriminatory potential to differentiate ALS from patients with symptoms mimicking ALS has not been investigated. We investigated the combination of brain and spine [18F]-FDG PET-CT for differential diagnosis between ALS and ALS mimics in a real-life clinical diagnostic setting.
Methods
Patients with a suspected diagnosis of ALS (n = 98; 64.8 ± 11 years; 61 M) underwent brain and spine [18F]-FDG PET-CT scans. In 62 patients, ALS diagnosis was confirmed (67.8 ± 10 years; 35 M) after longitudinal follow-up (average 18.1 ± 8.4 months). In 23 patients, another disease was diagnosed (ALS mimics, 60.9 ± 12.9 years; 17 M) and 13 had a variant motor neuron disease, primary lateral sclerosis (PLS; n = 4; 53.6 ± 2.5 years; 2 M) and progressive muscular atrophy (PMA; n = 9; 58.4 ± 7.3 years; 7 M). Spine metabolism was determined after manual and automated segmentation. VOI- and voxel-based comparisons were performed. Moreover, a support vector machine (SVM) approach was applied to investigate the discriminative power of regional brain metabolism, spine metabolism and the combination of both.
Results
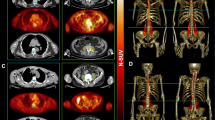
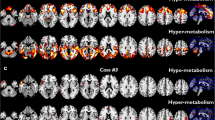
Brain metabolism was very similar between ALS mimics and ALS, whereas cervical and thoracic spine metabolism was significantly different (in standardised uptake values; cervical: ALS 2.1 ± 0.5, ALS mimics 1.9 ± 0.4; thoracic: ALS 1.8 ± 0.3, ALS mimics 1.5 ± 0.3). As both brain and spine metabolisms were very similar between ALS mimics and PLS/PMA, groups were pooled for accuracy analyses. Mean discrimination accuracy was 65.4%, 80.0% and 81.5%, using only brain metabolism, using spine metabolism and using both, respectively.
Conclusion
The combination of brain and spine FDG PET-CT with SVM classification is useful as discriminative biomarker between ALS and ALS mimics in a real-life clinical setting.





Similar content being viewed by others
References
Salameh JS, Brown RH Jr, Berry JD. Amyotrophic lateral sclerosis: review. Semin Neurol. 2015;35(4):469–76.
Paganoni S, Macklin EA, Lee A, Murphy A, Chang J, Zipf A, et al. Diagnostic timelines and delays in diagnosing amyotrophic lateral sclerosis (ALS). Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):453–6.
Pagani M, Oberg J, De Carli F, Calvo A, Moglia C, Canosa A, et al. Metabolic spatial connectivity in amyotrophic lateral sclerosis as revealed by independent component analysis. Hum Brain Mapp. 2016;37(3):942–53.
Van Laere K, Vanhee A, Verschueren J, De Coster L, Driesen A, Dupont P, et al. Value of 18fluorodeoxyglucose-positron-emission tomography in amyotrophic lateral sclerosis: a prospective study. JAMA Neurol. 2014;71(5):553–61.
Van Weehaeghe D, Ceccarini J, Delva A, Robberecht W, Van Damme P, Van Laere K. Prospective validation of 18F-FDG brain PET discriminant analysis methods in the diagnosis of amyotrophic lateral sclerosis. J Nucl Med. 2016;57(8):1238–43.
Wheeler-Kingshott CA, Stroman PW, Schwab JM, Bacon M, Bosma R, Brooks J, et al. The current state-of-the-art of spinal cord imaging: applications. Neuroimage. 2014;84:1082–93.
Marini C, Cistaro A, Campi C, Calvo A, Caponnetto C, Nobili FM, et al. A PET/CT approach to spinal cord metabolism in amyotrophic lateral sclerosis. Eur J Nucl Med Mol Imaging. 2016;43(11):2061–71.
Branco LM, De Albuquerque M, De Andrade HM, Bergo FP, Nucci A, Franca MC Jr. Spinal cord atrophy correlates with disease duration and severity in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(1–2):93–7.
El Mendili MM, Cohen-Adad J, Pelegrini-Issac M, Rossignol S, Morizot-Koutlidis R, Marchand-Pauvert V, et al. Multi-parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PLoS One. 2014;9(4):e95516.
Grolez G, Kyheng M, Lopes R, Moreau C, Timmerman K, Auger F, et al. MRI of the cervical spinal cord predicts respiratory dysfunction in ALS. Sci Rep. 2018;8(1):1828.
Costa J, Swash M, de Carvalho M. Awaji criteria for the diagnosis of amyotrophic lateral sclerosis: a systematic review. Arch Neurol. 2012;69(11):1410–6.
Schrooten M, Smetcoren C, Robberecht W, Van Damme P. Benefit of the Awaji diagnostic algorithm for amyotrophic lateral sclerosis: a prospective study. Ann Neurol. 2011;70(1):79–83.
Balendra R, Jones A, Jivraj N, Knights C, Ellis CM, Burman R, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(3–4):279–84.
van Weehaeghe D, Ceccarini J, Willekens SM, de Vocht J, van Damme P, van Laere K. Is there a glucose metabolic signature of spreading TDP-43 pathology in amyotrophic lateral sclerosis? Q J Nucl Med Mol Imaging. 2017.
Hearst MA. Support vector machines. IEEE Intell Syst App. 1998;13(4):18–21.
van den Hoff J, Lougovski A, Schramm G, Maus J, Oehme L, Petr J, et al. Correction of scan time dependence of standard uptake values in oncological PET. EJNMMI Res. 2014;4(1):18.
Ronneberger O, Fischer P, Brox T. U-net: convolutional networks for biomedical image segmentation. Lect Notes Comput Sc. 2015;9351:234–41.
Srivastava N, Hinton G, Krizhevsky A, Sutskever I, Salakhutdinov R. Dropout: a simple way to prevent neural networks from overfitting. J Mach Learn Res. 2014;15:1929–58.
De Leener B, Levy S, Dupont SM, Fonov VS, Stikov N, Collins DL, et al. SCT: spinal cord toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017;145:24–43.
De Leener B, Mangeat G, Dupont S, Martin AR, Callot V, Stikov N, et al. Topologically preserving straightening of spinal cord MRI. J Magn Reson Imaging. 2017;46(4):1209–19.
Tustison NJ, Cook PA, Klein A, Song G, Das SR, Duda JT, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage. 2014;99:166–79.
Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26–41.
Lai TH, Liu RS, Yang BH, Wang PS, Lin KP, Lee YC, et al. Cerebral involvement in spinal and bulbar muscular atrophy (Kennedy’s disease): a pilot study of PET. J Neurol Sci. 2013;335(1–2):139–44.
Pedersen BK. Physical activity and muscle-brain crosstalk. Nat Rev Endocrinol. 2019;15(7):383–92.
Clark BC, Mahato NK, Nakazawa M, Law TD, Thomas JS. The power of the mind: the cortex as a critical determinant of muscle strength/weakness. J Neurophysiol. 2014;112(12):3219–26.
Shimada H, Ishii K, Ishiwata K, Oda K, Suzukawa M, Makizako H, et al. Gait adaptability and brain activity during unaccustomed treadmill walking in healthy elderly females. Gait Posture. 2013;38(2):203–8.
Tashiro M, Itoh M, Fujimoto T, Fujiwara T, Ota H, Kubota K, et al. 18F-FDG PET mapping of regional brain activity in runners. J Sports Med Phys Fitness. 2001;41(1):11–7.
Watson N, Ji X, Yasuhara T, Date I, Kaneko Y, Tajiri N, et al. No pain, no gain: lack of exercise obstructs neurogenesis. Cell Transplant. 2015;24(4):591–7.
Marini C, Morbelli S, Cistaro A, Campi C, Caponnetto C, Bauckneht M, et al. Interplay between spinal cord and cerebral cortex metabolism in amyotrophic lateral sclerosis. Brain. 2018;141(8):2272–9.
Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39(2):251–9.
Brettschneider J, Del Tredici K, Toledo JB, Robinson JL, Irwin DJ, Grossman M, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol. 2013;74(1):20–38.
Feneberg E, Oeckl P, Steinacker P, Verde F, Barro C, Van Damme P, et al. Multicenter evaluation of neurofilaments in early symptom onset amyotrophic lateral sclerosis. Neurology. 2018;90(1):E22–30.
van der Burgh HK, Westeneng HJ, Meier JM, van Es MA, Veldink JH, Hendrikse J, et al. Cross-sectional and longitudinal assessment of the upper cervical spinal cord in motor neuron disease. Neuroimage Clin. 2019;24:101984.
Acknowledgements
The authors want to thank the nuclear medicine team for their contributions to the scanning and data handling, the PET radiopharmacy team and the medical physics team of UZ Leuven for their skilled contributions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PVD, KVL and MK are senior clinical investigators of the research foundation of Flandres (FWO). PVD is supported by the E. von Behring Chair for Neuromuscular and Neurodegenerative Disorders, the ALS Liga België and the KU Leuven funds ‘Een Hart voor ALS’, ‘Laeversfonds voor ALS Onderzoek’ and ‘the Valéry Perrier Race against ALS fund’. MD and DVW and PhD fellows from FWO. JDV is funded by the Klinische onderzoeks- en opleidingsraad (KOOR) of the University Hospitals Leuven. No other conflicts of interest were present.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
This retrospective study was approved by the local University Hospital Ethics Committee; because of its retrospective nature, the need for written consent was waived.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Rights and permissions
About this article
Cite this article
Van Weehaeghe, D., Devrome, M., Schramm, G. et al. Combined brain and spinal FDG PET allows differentiation between ALS and ALS mimics. Eur J Nucl Med Mol Imaging 47, 2681–2690 (2020). https://doi.org/10.1007/s00259-020-04786-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04786-y