Abstract
Purpose
Intercellular adhesion molecule-1 (ICAM-1, CD54) is an emerging therapeutic target for a variety of solid tumors including melanoma and anaplastic thyroid cancer (ATC). This study aims to develop an ICAM-1-targeted immuno-positron emission tomography (immunoPET) imaging strategy and assess its diagnostic value in melanoma and ATC models.
Methods
Flow cytometry was used to screen ICAM-1-positive melanoma and ATC cell lines. Melanoma and ATC models were established using A375 cell line and THJ-16T cell line, respectively. An ICAM-1-specific monoclonal antibody (R6-5-D6) and a nonspecific human IgG were radiolabeled with 64Cu and the diagnostic efficacies were interrogated in tumor-bearing mouse models. Biodistribution and fluorescent imaging studies were performed to confirm the specificity of the ICAM-1-targeted imaging probes.
Results
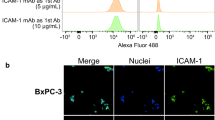
ICAM-1 was strongly expressed on melanoma and advanced thyroid cancer cell lines. 64Cu-NOTA-ICAM-1 immunoPET imaging efficiently delineated A375 melanomas with a peak tumor uptake of 21.28 ± 6.56 %ID/g (n = 5), significantly higher than that of 64Cu-NOTA-IgG (10.63 ± 2.58 %ID/g, n = 3). Moreover, immunoPET imaging with 64Cu-NOTA-ICAM-1 efficiently visualized subcutaneous and orthotopic ATCs with high clarity and contrast. Fluorescent imaging with IRDye 800CW-ICAM-1 also visualized orthotopic ATCs and the tumor uptake could be blocked by the ICAM-1 parental antibody R6-5-D6, indicating the high specificity of the developed probe. Finally, blocking with the human IgG prolonged the circulation of the 64Cu-NOTA-ICAM-1 in R2G2 mice without compromising the tumor uptake.
Conclusion
ICAM-1-targeted immunoPET imaging could characterize ICAM-1 expression in melanoma and ATC, which holds promise for optimizing ICAM-1-targeted therapies in the future.






Similar content being viewed by others
References
Reina M, Espel E. Role of LFA-1 and ICAM-1 in cancer. Cancers (Basel). 2017;9. https://doi.org/10.3390/cancers9110153.
Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-alpha-activated vascular endothelium under flow. Blood. 2005;106:584–92. https://doi.org/10.1182/blood-2004-12-4942.
Hayes SH, Seigel GM. Immunoreactivity of ICAM-1 in human tumors, metastases and normal tissues. Int J Clin Exp Pathol. 2009;2:553–60.
Hamai A, Meslin F, Benlalam H, Jalil A, Mehrpour M, Faure F, et al. ICAM-1 has a critical role in the regulation of metastatic melanoma tumor susceptibility to CTL lysis by interfering with PI3K/AKT pathway. Cancer Res. 2008;68:9854–64. https://doi.org/10.1158/0008-5472.CAN-08-0719.
Zhang P, Goodrich C, Fu C, Dong C. Melanoma upregulates ICAM-1 expression on endothelial cells through engagement of tumor CD44 with endothelial E-selectin and activation of a PKCalpha-p38-SP-1 pathway. FASEB J. 2014;28:4591–609. https://doi.org/10.1096/fj.11-202747.
Galore-Haskel G, Baruch EN, Berg AL, Barshack I, Zilinsky I, Avivi C, et al. Histopathological expression analysis of intercellular adhesion molecule 1 (ICAM-1) along development and progression of human melanoma. Oncotarget. 2017;8:99580–6. https://doi.org/10.18632/oncotarget.20884.
Buitrago D, Keutgen XM, Crowley M, Filicori F, Aldailami H, Hoda R, et al. Intercellular adhesion molecule-1 (ICAM-1) is upregulated in aggressive papillary thyroid carcinoma. Ann Surg Oncol. 2012;19:973–80. https://doi.org/10.1245/s10434-011-2029-0.
Min IM, Shevlin E, Vedvyas Y, Zaman M, Wyrwas B, Scognamiglio T, et al. CAR T therapy targeting ICAM-1 eliminates advanced human thyroid tumors. Clin Cancer Res. 2017;23:7569–83. https://doi.org/10.1158/1078-0432.CCR-17-2008.
Vedvyas Y, McCloskey JE, Yang Y, Min IM, Fahey TJ, Zarnegar R, et al. Manufacturing and preclinical validation of CAR T cells targeting ICAM-1 for advanced thyroid cancer therapy. Sci Rep. 2019;9:10634. https://doi.org/10.1038/s41598-019-46938-7.
James ML, Gambhir SS. A molecular imaging primer: modalities, imaging agents, and applications. Physiol Rev. 2012;92:897–965. https://doi.org/10.1152/physrev.00049.2010.
Guo P, Huang J, Wang L, Jia D, Yang J, Dillon DA, et al. ICAM-1 as a molecular target for triple negative breast cancer. Proc Natl Acad Sci U S A. 2014;111:14710–5. https://doi.org/10.1073/pnas.1408556111.
Bensch F, Smeenk MM, van Es SC, de Jong JR, Schroder CP, Oosting SF, et al. Comparative biodistribution analysis across four different (89)Zr-monoclonal antibody tracers-the first step towards an imaging warehouse. Theranostics. 2018;8:4295–304. https://doi.org/10.7150/thno.26370.
Bensch F, Brouwers AH, Lub-de Hooge MN, de Jong JR, van der Vegt B, Sleijfer S, et al. (89)Zr-trastuzumab PET supports clinical decision making in breast cancer patients, when HER2 status cannot be determined by standard work up. Eur J Nucl Med Mol Imaging. 2018;45:2300–6. https://doi.org/10.1007/s00259-018-4099-8.
Ulaner GA, Lyashchenko SK, Riedl C, Ruan S, Zanzonico PB, Lake D, et al. First-in-human human epidermal growth factor receptor 2-targeted imaging using (89)Zr-Pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med. 2018;59:900–6. https://doi.org/10.2967/jnumed.117.202010.
Wei W, Ni D, Ehlerding EB, Luo Q-Y, Cai W. PET imaging of receptor tyrosine kinases in cancer. Mol Cancer Ther. 2018;17:1625–36. https://doi.org/10.1158/1535-7163.mct-18-0087.
Lamberts LE, Menke-van der Houven van Oordt CW, ter Weele EJ, Bensch F, Smeenk MM, Voortman J, et al. ImmunoPET with anti-mesothelin antibody in patients with pancreatic and ovarian cancer before anti-mesothelin antibody-drug conjugate treatment. Clin Cancer Res. 2016;22:1642–52. https://doi.org/10.1158/1078-0432.CCR-15-1272.
Wei W, Jiang D, Ehlerding EB, Luo Q, Cai W. Noninvasive PET imaging of T cells. Trends Cancer. 2018;4:359–73. https://doi.org/10.1016/j.trecan.2018.03.009.
Cai W, Rao J, Gambhir SS, Chen X. How molecular imaging is speeding up antiangiogenic drug development. Mol Cancer Ther. 2006;5:2624–33. https://doi.org/10.1158/1535-7163.MCT-06-0395.
Wei W, Jiang D, Ehlerding EB, Barnhart TE, Yang Y, Engle JW, et al. CD146-targeted multimodal image-guided photoimmunotherapy of melanoma. Adv Sci (Weinh). 2019;6:1801237. https://doi.org/10.1002/advs.201801237.
Wei W, Jiang D, Rosenkrans ZT, Barnhart TE, Engle JW, Luo Q, et al. HER2-targeted multimodal imaging of anaplastic thyroid cancer. Am J Cancer Res. 2019;9:2413–27.
Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, et al. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid. 2009;19:1077–84. https://doi.org/10.1089/thy.2009.0055.
Jin Y, Liu M, Sa R, Fu H, Cheng L, Chen L. Mouse models of thyroid cancer: bridging pathogenesis and novel therapeutics. Cancer Lett. 2020;469:35–53. https://doi.org/10.1016/j.canlet.2019.09.017.
Yang Y, Hernandez R, Rao J, Yin L, Qu Y, Wu J, et al. Targeting CD146 with a 64Cu-labeled antibody enables in vivo immunoPET imaging of high-grade gliomas. Proc Natl Acad Sci U S A. 2015;112:E6525–34. https://doi.org/10.1073/pnas.1502648112.
Cohen R, Vugts DJ, Stigter-van Walsum M, Visser GW, van Dongen GA. Inert coupling of IRDye800CW and zirconium-89 to monoclonal antibodies for single- or dual-mode fluorescence and PET imaging. Nat Protoc. 2013;8:1010–8. https://doi.org/10.1038/nprot.2013.054.
Wei W, Ehlerding EB, Lan X, Luo Q, Cai W. PET and SPECT imaging of melanoma: the state of the art. Eur J Nucl Med Mol Imaging. 2018;45:132–50. https://doi.org/10.1007/s00259-017-3839-5.
Sharma SK, Chow A, Monette S, Vivier D, Pourat J, Edwards KJ, et al. Fc-mediated anomalous biodistribution of therapeutic antibodies in immunodeficient mouse models. Cancer Res. 2018;78:1820–32. https://doi.org/10.1158/0008-5472.Can-17-1958.
Pereira PMR, Abma L, Henry KE, Lewis JS. Imaging of human epidermal growth factor receptors for patient selection and response monitoring - from PET imaging and beyond. Cancer Lett. 2018;419:139–51. https://doi.org/10.1016/j.canlet.2018.01.052.
Lin NU, Bellon JR, Winer EP. CNS metastases in breast cancer. J Clin Oncol. 2004;22:3608–17. https://doi.org/10.1200/JCO.2004.01.175.
Pandit-Taskar N, O'Donoghue JA, Beylergil V, Lyashchenko S, Ruan S, Solomon SB, et al. (8)(9)Zr-huJ591 immuno-PET imaging in patients with advanced metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2014;41:2093–105. https://doi.org/10.1007/s00259-014-2830-7.
Moek KL, Giesen D, Kok IC, de Groot DJA, Jalving M, Fehrmann RSN, et al. Theranostics using antibodies and antibody-related therapeutics. J Nucl Med. 2017;58:83S–90S. https://doi.org/10.2967/jnumed.116.186940.
Burvenich IJG, Parakh S, Parslow AC, Lee ST, Gan HK, Scott AM. Receptor occupancy imaging studies in oncology drug development. AAPS J. 2018;20:43. https://doi.org/10.1208/s12248-018-0203-z.
Lamberts LE, Williams SP, Terwisscha van Scheltinga AG, Lub-de Hooge MN, Schroder CP, Gietema JA, et al. Antibody positron emission tomography imaging in anticancer drug development. J Clin Oncol. 2015;33:1491–504. https://doi.org/10.1200/JCO.2014.57.8278.
Wu X, Guo R, Wang Y, Cunningham PN. The role of ICAM-1 in endotoxin-induced acute renal failure. Am J Physiol Renal Physiol. 2007;293:F1262–71. https://doi.org/10.1152/ajprenal.00445.2006.
Mar D, Gharib SA, Zager RA, Johnson A, Denisenko O, Bomsztyk K. Heterogeneity of epigenetic changes at ischemia/reperfusion- and endotoxin-induced acute kidney injury genes. Kidney Int. 2015;88:734–44. https://doi.org/10.1038/ki.2015.164.
Zumwalde NA, Domae E, Mescher MF, Shimizu Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J Immunol. 2013;191:3681–93. https://doi.org/10.4049/jimmunol.1201954.
Hailemichael Y, Woods A, Fu T, He Q, Nielsen MC, Hasan F, et al. Cancer vaccine formulation dictates synergy with CTLA-4 and PD-L1 checkpoint blockade therapy. J Clin Invest. 2018;128:1338–54. https://doi.org/10.1172/JCI93303.
Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV. Signaling by antibodies: recent progress. Annu Rev Immunol. 2017;35:285–311. https://doi.org/10.1146/annurev-immunol-051116-052433.
Warnders FJ, Lub-de Hooge MN, de Vries EGE, Kosterink JGW. Influence of protein properties and protein modification on biodistribution and tumor uptake of anticancer antibodies, antibody derivatives, and non-Ig scaffolds. Med Res Rev. 2018;38:1837–73. https://doi.org/10.1002/med.21498.
Dalziel M, Crispin M, Scanlan CN, Zitzmann N, Dwek RA. Emerging principles for the therapeutic exploitation of glycosylation. Science. 2014;343:1235681. https://doi.org/10.1126/science.1235681.
Pincetic A, Bournazos S, DiLillo DJ, Maamary J, Wang TT, Dahan R, et al. Type I and type II Fc receptors regulate innate and adaptive immunity. Nat Immunol. 2014;15:707–16. https://doi.org/10.1038/ni.2939.
Arlauckas SP, Garris CS, Kohler RH, Kitaoka M, Cuccarese MF, Yang KS, et al. In vivo imaging reveals a tumor-associated macrophage-mediated resistance pathway in anti-PD-1 therapy. Sci Transl Med. 2017;9. https://doi.org/10.1126/scitranslmed.aal3604.
Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, et al. Anti-CTLA-4 therapy requires an Fc domain for efficacy. Proc Natl Acad Sci U S A. 2018;115:3912–7. https://doi.org/10.1073/pnas.1801524115.
Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33:649–63 e4. https://doi.org/10.1016/j.ccell.2018.02.010.
Dahan R, Sega E, Engelhardt J, Selby M, Korman AJ, Ravetch JV. FcgammaRs modulate the anti-tumor activity of antibodies targeting the PD-1/PD-L1 axis. Cancer Cell. 2015;28:285–95. https://doi.org/10.1016/j.ccell.2015.08.004.
Chakravarty R, Goel S, Cai W. Nanobody: the “magic bullet” for molecular imaging? Theranostics. 2014;4:386–98. https://doi.org/10.7150/thno.8006.
Keyaerts M, Xavier C, Heemskerk J, Devoogdt N, Everaert H, Ackaert C, et al. Phase I study of 68Ga-HER2-nanobody for PET/CT assessment of HER2 expression in breast carcinoma. J Nucl Med. 2016;57:27–33. https://doi.org/10.2967/jnumed.115.162024.
Xing Y, Chand G, Liu C, Cook GJR, O’Doherty J, Zhao L, et al. Early phase I study of a (99m)Tc-labeled anti-programmed death ligand-1 (PD-L1) single-domain antibody in SPECT/CT assessment of PD-L1 expression in non-small cell lung cancer. J Nucl Med. 2019;60:1213–20. https://doi.org/10.2967/jnumed.118.224170.
Acknowledgments
We appreciate Dr. Hao Yang and Prof. Gang Huang (Shanghai Key Laboratory of Molecular Imaging, Shanghai University of Medicine and Health Sciences) for their help in acquiring fluorescent imaging data.
Funding
This work was supported, in part, by the University of Wisconsin – Madison, the National Institutes of Health (P30CA014520), and the Natural Science Foundation of China (81771858).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants performed by any of the authors. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Preclinical Imaging
Electronic supplementary material
ESM 1
(DOCX 1092 kb).
Rights and permissions
About this article
Cite this article
Wei, W., Jiang, D., Lee, H.J. et al. Development and characterization of CD54-targeted immunoPET imaging in solid tumors. Eur J Nucl Med Mol Imaging 47, 2765–2775 (2020). https://doi.org/10.1007/s00259-020-04784-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04784-0