Abstract
Purpose
The aim of this study was to investigate metabolic and textural parameters from pretreatment [18F]FDG PET/CT scans for the prediction of neoadjuvant radiation chemotherapy response and 3-year disease-free survival (DFS) in patients with locally advanced rectal cancer (LARC).
Methods
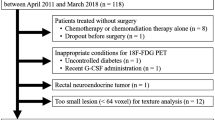
We performed a retrospective review of 74 patients diagnosed with LARC who were initially examined with [18F]FDG PET/CT, and who underwent neoadjuvant radiation chemotherapy followed by complete resection. The standardized uptake value (mean, peak, and maximum), metabolic volume (MV), and total lesion glycolysis of rectal cancer lesions were calculated using the isocontour method with various thresholds. Using three-dimensional textural analysis, about 50 textural features were calculated for PET images. Response to neoadjuvant radiation chemotherapy, as assessed by histological tumour regression grading (TRG) after surgery and 3-year DFS, was evaluated using univariate/multivariate binary logistic regression and univariate/multivariate Cox regression analyses.
Results
MVs calculated using the thresholds mean standardized uptake value of the liver + two standard deviations (SDs), and mean standard uptake of the liver + three SDs were significantly associated with TRG. Textural parameters from histogram-based and co-occurrence analysis were significantly associated with TRG. However, multivariate analysis revealed that none of these parameters had any significance. On the other hand, MV calculated using various thresholds was significantly associated with 3-year DFS, and MV calculated using a higher threshold tended to be more strongly associated with 3-year DFS. In addition, textural parameters including kurtosis of the absolute gradient (GrKurtosis) were significantly associated with 3-year DFS. Multivariate analysis revealed that GrKurtosis could be a prognostic factor for 3-year DFS.
Conclusion
Metabolic and textural parameters from initial [18F]FDG PET/CT scans could be indexes to assess tumour heterogeneity for the prediction of neoadjuvant radiation chemotherapy response and recurrence in LARC.


Similar content being viewed by others
References
Guillem JG, Chessin DB, Cohen AM, Shia J, Mazumdar M, Enker W, et al. Long-term oncologic outcome following preoperative combined modality therapy and total mesorectal excision of locally advanced rectal cancer. Ann Surg. 2005;241(5):829–36.
Kaminsky-Forrett M-C, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, et al. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42(5):935–41.
Theodoropoulos G, Wise W, Padmanabhan A, Kerner B, Taylor C, Aguilar P, et al. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis Colon Rectum. 2002;45(7):895–903.
García-Aguilar J, de Anda EH, Sirivongs P, Lee S-H, Madoff RD, Rothenberger DA. A pathologic complete response to preoperative chemoradiation is associated with lower local recurrence and improved survival in rectal cancer patients treated by mesorectal excision. Dis Colon Rectum. 2003;46(3):298–304.
Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo L-J, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–44.
Rödel C, Martus P, Papadoupolos T, Füzesi L, Klimpfinger M, Fietkau R, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. Clin Oncol. 2005;23(34):8688–96.
Brown G, Davies S, Williams G, Bourne M, Newcombe R, Radcliffe A, et al. Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging? Br J Cancer. 2004;91(1):23–9.
Kahn H, Alexander A, Rakinic J, Nagle D, Fry R. Preoperative staging of irradiated rectal cancers using digital rectal examination, computed tomography, endorectal ultrasound, and magnetic resonance imaging does not accurately predict T0, N0 pathology. Dis Colon Rectum. 1997;40(2):140–4.
Suppiah A, Hunter I, Cowley J, Garimella V, Cast J, Hartley J, et al. Magnetic resonance imaging accuracy in assessing tumour down‐staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11(3):249–53.
Calvo FA, Domper M, Matute R, Martinez-Lazaro R, Arranz JA, Desco M, et al. 18F-FDG positron emission tomography staging and restaging in rectal cancer treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys. 2004;58(2):528–35.
Capirci C, Rampin L, Erba PA, Galeotti F, Crepaldi G, Banti E, et al. Sequential FDG-PET/CT reliably predicts response of locally advanced rectal cancer to neo-adjuvant chemo-radiation therapy. Eur J Nucl Med Mol Imaging. 2007;34(10):1583–93.
Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Guillem JG, Moore HG, Akhurst T, Klimstra DS, Ruo L, Mazumdar M, et al. Sequential preoperative fluorodeoxyglucose-positron emission tomography assessment of response to preoperative chemoradiation: a means for determining longterm outcomes of rectal cancer. J Am Coll Surg. 2004;199(1):1–7.
Konski A, Hoffman J, Sigurdson E, Haluszka O, Engstrom P, Cheng JD, et al. Can molecular imaging predict response to preoperative chemoradiation in patients with rectal cancer? A Fox Chase Cancer Center prospective experience. Semin Oncol. 2005;32(6 Suppl 9):S63–7.
Chicklore S, Goh V, Siddique M, Roy A, Marsden PK, Cook GJ. Quantifying tumour heterogeneity in 18F-FDG PET/CT imaging by texture analysis. Eur J Nucl Med Mol Imaging. 2013;40(1):133–40.
Ha S, Choi H, Cheon GJ, Kang KW, Chung J-K, Kim EE, et al. Autoclustering of non-small cell lung carcinoma subtypes on 18F-FDG PET using texture analysis: a preliminary result. Nucl Med Mol Imaging. 2014;48(4):278–86.
Tixier F, Le Rest CC, Hatt M, Albarghach N, Pradier O, Metges J-P, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–78.
Bundschuh RA, Dinges J, Neumann L, Seyfried M, Zsótér N, Papp L, et al. Textural parameters of tumor heterogeneity in 18F-FDG PET/CT for therapy response assessment and prognosis in patients with locally advanced rectal cancer. J Nucl Med. 2014;55(6):891–7.
Yoo S, Kim S, Lee K-H, Jeong CW, Youn SW, Park KU, et al. Electronically implemented clinical indicators based on a data warehouse in a tertiary hospital: its clinical benefit and effectiveness. Int J Med Inform. 2014;83(7):507–16.
Strzelecki M, Szczypinski P, Materka A, Klepaczko A. A software tool for automatic classification and segmentation of 2D/3D medical images. Nucl Instrum Methods Phys Res A. 2013;702:137–40.
Szczypiński PM, Strzelecki M, Materka A. MaZda - a software for texture analysis. International Symposium on Information Technology Convergence. ISITC 2007. 23–24 November 2007, Jeonju, Korea. p. 245–9. doi:10.1109/ISITC.2007.15.
Szczypiński PM, Strzelecki M, Materka A, Klepaczko A. MaZda – a software package for image texture analysis. Comput Methods Programs Biomed. 2009;94(1):66–76.
Materka A SMPSs. MaZda manual. 2006.
Oku S, Nakagawa K, Momose T, Kumakura Y, Abe A, Watanabe T, et al. FDG-PET after radiotherapy is a good prognostic indicator of rectal cancer. Ann Nucl Med. 2002;16(6):409–16.
Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the MD Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44(5):1027–38.
Willett CG, Warland G, Coen J, Shellito PC, Compton CC. Rectal cancer: the influence of tumor proliferation on response to preoperative irradiation. Int J Radiat Oncol Biol Phys. 1995;32(1):57–61.
Henriksson E, Kjellen E, Wahlberg P, Ohlsson T, Wennerberg J, Brun E. 2-Deoxy-2-[18F] fluoro-D-glucose uptake and correlation to intratumoral heterogeneity. Anticancer Res. 2007;27(4B):2155–9.
Halfpenny W, Hain S, Biassoni L, Maisey M, Sherman J, McGurk M. FDG-PET. a possible prognostic factor in head and neck cancer. Br J Cancer. 2002;86(4):512–6.
Higashi K, Ueda Y, Ayabe K, Sakurai A, Seki H, Nambu Y, et al. FDG PET in the evaluation of the aggressiveness of pulmonary adenocarcinoma: correlation with histopathological features. Nucl Med Commun. 2000;21(8):707–14.
Ngeow J, Quek R, Ng D, Hee S, Tao M, Lim L, et al. High SUV uptake on FDG–PET/CT predicts for an aggressive B-cell lymphoma in a prospective study of primary FDG-PET/CT staging in lymphoma. Ann Oncol. 2009;20(9):1543–7.
Pasquali C, Rubello D, Sperti C, Gasparoni P, Liessi G, Chierichetti F, et al. Neuroendocrine tumor imaging: can 18F-fluorodeoxyglucose positron emission tomography detect tumors with poor prognosis and aggressive behavior? World J Surg. 1998;22(6):588–92.
Castellano G, Bonilha L, Li LM, Cendes F. Texture analysis of medical images. Clin Radiol. 2004;59(12):1061–9.
van Baardwijk A, Dooms C, van Suylen RJ, Verbeken E, Hochstenbag M, Dehing-Oberije C, et al. The maximum uptake of (18)F-deoxyglucose on positron emission tomography scan correlates with survival, hypoxia inducible factor-1alpha and GLUT-1 in non-small cell lung cancer. Eur J Cancer. 2007;43(9):1392–8.
Yamada T, Uchida M, Kwang-Lee K, Kitamura N, Yoshimura T, Sasabe E, et al. Correlation of metabolism/hypoxia markers and fluorodeoxyglucose uptake in oral squamous cell carcinomas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2012;113(4):464–71.
Hall EJ, Giaccia A. The oxygen effect and reoxygenation. Radiobiol Radiologist. 1994;4:133–52.
Pires A, Rusinek H, Suh J, Naidich DP, Pass H, et al. Clustering of lung adenocarcinomas classes using automated texture analysis on CT images. Proc. SPIE 8669, Medical Imaging 2013: Image Processing, 866925. doi:10.1117/12.2007154
Hofheinz R-D, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13(6):579–88.
Orlhac F, Soussan M, Maisonobe J-A, Garcia CA, Vanderlinden B, Buvat I. Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55(3):414–22.
Compliance with ethical standards
ᅟ
Funding
This research was supported by Korea Mouse Phenotyping Project (2013M3A9D5072560) of the Ministry of Science, ICT and Future Planning through the National Research Foundation.
Conflicts of interest
None.
Ethical approval
All procedures were performed in accordance with the principles of the 1975 Declaration of Helsinki (2000 revision). The study design and exemption from informed consent were approved by the Institutional Review Board of Seoul National University Bundang Hospital.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bang, JI., Ha, S., Kang, SB. et al. Prediction of neoadjuvant radiation chemotherapy response and survival using pretreatment [18F]FDG PET/CT scans in locally advanced rectal cancer. Eur J Nucl Med Mol Imaging 43, 422–431 (2016). https://doi.org/10.1007/s00259-015-3180-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-015-3180-9