Abstract
Purpose
11C-labelled 1-[(S)-1-(2,3-dihydrobenzo[1,2]dioxin-2-yl)methyl]-4-(3-methoxy-methylpyridin-2-yl)-piperazine (11C-ORM-13070) is a novel PET tracer for imaging of α2C-adrenoceptors in the human brain. Brain α2C-adrenoceptors may be therapeutic targets in several neuropsychiatric disorders, including depression, schizophrenia and Alzheimer’s disease. To validate the use of 11C-ORM-13070 in humans, we investigated its radiometabolism, pharmacokinetics, whole-body distribution and radiation dose.
Methods
Radiometabolism was studied in a test–retest setting in six healthy men. After intravenous injection of 11C-ORM-13070, blood samples were drawn over 60 min. Plasma samples were analysed by radio-HPLC for intact tracer and its radioactive metabolites. Metabolite-corrected plasma time–activity curves were used for calculation of pharmacokinetics. In a separate group of 12 healthy men, the whole-body distribution of 11C-ORM-13070 and radiation exposure were investigated by dynamic PET/CT imaging without blood sampling.
Results
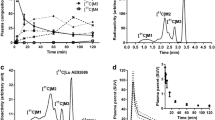
Two radioactive metabolites of 11C-ORM-13070 were detected in human arterial plasma. The proportion of unchanged 11C-ORM-13070 decreased from 81 ± 4 % of total radioactivity at 4 min after tracer injection to 23 ± 4 % at 60 min. At least one of the radioactive metabolites penetrated into red blood cells, while the parent tracer remained in plasma. The apparent elimination rate constant and corresponding half-life of unchanged 11C-ORM-13070 in arterial plasma were 0.0117 ± 0.0056 min−1 and 73.6 ± 35.8 min, respectively. The organs with the highest absorbed doses were the liver (12 μSv/MBq), gallbladder wall (12 μSv/MBq) and pancreas (9.1 μSv/MBq). The mean effective dose was 3.9 μSv/MBq, with a range of 3.6 – 4.2 μSv/MBq.
Conclusion
11C-ORM-13070 was rapidly metabolized in human subjects after intravenous injection. The effective radiation dose of 11C-ORM-13070 was in the same range as that of other 11C-labelled brain receptor tracers. An injection of 500 MBq of 11C-ORM-13070 would expose a subject to 2.0 mSv of radiation. This supports the use of 11C-ORM-13070 in repeated PET scans, for example, in receptor occupancy trials with novel drug candidates.







Similar content being viewed by others
References
Scheinin M, Sallinen J, Haapalinna A. Evaluation of the alpha2C-adrenoceptor as a neuropsychiatric drug target – studies in transgenic mouse models. Life Sci. 2001;68:2277–85.
Arponen E, Helin S, Marjamäki P, Grönroos T, Någren K, Ingman K, et al. Radiosynthesis of a new alpha-2C-adrenoceptor tracer [11C]ORM-13070 and its preclinical evaluation in rats [abstract]. NeuroImage. 2010;52:S125.
Scheibner J, Trendelenburg AU, Hein L, Starke K. Alpha2-adrenoceptors modulating neuronal serotonin release: a study in alpha2-adrenoceptor subtype-deficient mice. Br J Pharmacol. 2001;132:925–33.
Knaus AE, Muthig V, Schickinger S, Moura E, Beetz N, Gilsbach R, et al. Alpha2-adrenoceptor subtypes – unexpected functions for receptors and ligands derived from gene-targeted mouse models. Neurochem Int. 2007;51:277–81.
Holmberg M, Fagerholm V, Scheinin M. Regional distribution of alpha(2C)-adrenoceptors in brain and spinal cord of control mice and transgenic mice overexpessing the alpha(2C)-subtype: an autoradiographic study with [3H]RX821002 and [3H]rauwolscine. Neuroscience. 2003;117:875–98.
Nicholas AP, Pieribone V, Hökfelt T. Distributions of mRNAs for alpha2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol. 1993;328:575–94.
Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, et al. Distribution of alpha 2-adrenergic receptor subtype gene expression in rat brain. Brain Res Mol Brain Res. 1994;21:133–49.
Fagerholm V, Philipp M, Hein L, Scheinin M. [Ethyl-3H]RS-79948-197 alpha2-adrenoceptor autoradiography – validation in alpha2-adrenoceptor knockout mice. Eur J Pharmacol. 2004;497:301–9.
Arponen E, Helin S, Marjamäki P, Grönroos T, Holm P, Löyttyniemi E, Någren K, Scheinin M, Haaparanta-Solin M, Sallinen J, Solin O. A PET tracer for brain α2C-adrenoceptors, 11C-ORM-13070: radiosynthesis and preclinical evaluation in rats and knockout mice. J Nucl Med. 2014. doi:10.2967/jnumed.113.135574.
Teräs M, Tolvanen T, Johansson JJ, Williams JJ, Knuuti J. Performance of the new generation of whole-body PET/CT scanners: Discovery STE and Discovery VCT. Eur J Nucl Med Mol Imaging. 2007;34:1683–92.
Valentin J, editor. ICRP Publication 89: Basic anatomical and physiological data for use in radiological protection: reference values. Oxford: Pergamon; 2002.
Stabin MG, Sparks RB, Crowe EB. OLINDA/EXM: the second generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7.
Siegel JA, Thomas SR, Stubbs JB, Stabin MG, Hays MT, Koral KF, et al. MIRD pamphlet no. 16: Techniques for quantitative radiopharmaceutical biodistribution data acquisition and analysis for use in human radiation dose estimates. J Nucl Med. 1999;40 Suppl:37S–61S.
Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 2007;27:369–77.
Fagerholm V, Rokka J, Nyman L, Sallinen J, Tiihonen J, Tupala E, et al. Autoradiographic characterization of alpha(2C)-adrenoceptors in the human striatum. Synapse. 2008;62:508–15.
International Commission on Radiological Protection. Radiation dose to patients from radiopharmaceuticals. Addendum 6 to ICRP Publication 53. Oxford: Pergamon; 2002.
Acknowledgments
The professional assistance of the staff at Turku PET Centre is greatly appreciated. Dr. Robert M. Badeau, Ph.D., of the Turku University Language Centre provided excellent assistance with the linguistic editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM2 (MPEG 110733 kb)
Rights and permissions
About this article
Cite this article
Luoto, P., Suilamo, S., Oikonen, V. et al. 11C-ORM-13070, a novel PET ligand for brain α2C-adrenoceptors: radiometabolism, plasma pharmacokinetics, whole-body distribution and radiation dosimetry in healthy men. Eur J Nucl Med Mol Imaging 41, 1947–1956 (2014). https://doi.org/10.1007/s00259-014-2782-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-014-2782-y