Abstract
Objective
To determine the value of CT and dynamic contrast-enhanced (DCE-)MRI for monitoring denosumab therapy of giant cell tumors of bone (GCTB) by correlating it to histopathology.
Materials and methods
Patients with GCTB under denosumab treatment and monitored with CT and (DCE-)MRI (2012-2021) were retrospectively included. Imaging and (semi-)quantitative measurements were used to assess response/relapse. Tissue samples were analyzed using computerized segmentation for vascularization and number of neoplastic and giant cells. Pearson’s correlation/Spearman’s rank coefficient and Kruskal-Wallis tests were used to assess correlations between histopathology and radiology.
Results
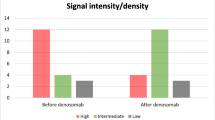
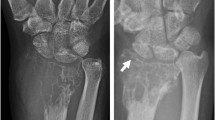
Six patients (28 ± 8years; five men) were evaluated. On CT, good responders showed progressive re-ossification (+7.8HU/month) and cortical remodeling (woven bone). MRI showed an SI decrease relative to muscle on T1-weighted (−0.01 A.U./month) and on fat-saturated T2-weighted sequences (−0.03 A.U./month). Time-intensity-curves evolved from a type IV with high first pass, high amplitude, and steep wash-out to a slow type II. An increase in time-to-peak (+100%) and a decrease in Ktrans (−71%) were observed. This is consistent with microscopic examination, showing a decrease of giant cells (−76%), neoplastic cells (-63%), and blood vessels (−28%). There was a strong statistical significant inverse correlation between time-to-peak and microvessel density (ρ = −0.9, p = 0.01). Significantly less neoplastic (p = 0.03) and giant cells (p = 0.04) were found with a time-intensity curve type II, compared to a type IV. Two patients showed relapse after initial good response when stopping denosumab. Inverse imaging and pathological findings were observed.
Conclusion
CT and (DCE-)MRI show a good correlation with pathology and allow adequate evaluation of response to denosumab and detection of therapy failure.






Similar content being viewed by others
Data availability
Data generated or analyzed during the study are available upon reasonable request.
References
Chawla S, Blay J-Y, Rutkowski P, Le Cesne A, Reichardt P, Gelderblom H, et al. Denosumab in patients with giant-cell tumour of bone: a multicentre, open-label, phase 2 study. Lancet Oncol. 2019;20(12):1719–29.
WHO Classification of Tumours Editorial Board. Soft Tissue and Bone Tumours: WHO Classification of Tumours (Medicine). 5th ed. International Agency for Research on Cancer; 2020.
Santini E, Kalil RK, Franco Bertoni Y-KP. Tumors and tumorlike lesions of bone, vol. 25. Springer; 1994. p. 593–3.
Presneau N, Baumhoer D, Behjati S, Pillay N, Tarpey P, Campbell PJ, et al. Diagnostic value of H3F3A mutations in giant cell tumour of bone compared to osteoclast-rich mimics. J Pathol Clin Res [Internet]. 2015;1(2):113–23. https://doi.org/10.1002/cjp2.13.
Zhang R, Ma T, Qi D, Zhao M, Hu T, Zhang G. Short-term preoperative denosumab with surgery in unresectable or recurrent giant cell tumor of bone. Orthop Surg. 2019;11(6):1101–8.
Lipplaa A, Dijkstra S, Gelderblom H. Challenges of denosumab in giant cell tumor of bone, and other giant cell-rich tumors of bone. Curr Opin Oncol. 2019;31(4):329–35.
van der Heijden L, Dijkstra PDS, Blay J-Y, Gelderblom H. Giant cell tumour of bone in the denosumab era. Eur J Cancer. 2017;77:75–83.
Mak IWY, Evaniew N, Popovic S, Tozer R, Ghert M. A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am. 2014;96(15):e127.
Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay J-Y, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11(3):275–80.
Chawla S, Henshaw R, Seeger L, Choy E, Blay J-Y, Ferrari S, et al. Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol. 2013;14(9):901–8.
Gossai N, Hilgers MV, Polgreen LE, Greengard EG. Critical hypercalcemia following discontinuation of denosumab therapy for metastatic giant cell tumor of bone. Pediatr Blood Cancer. 2015;62(6):1078–80.
van Langevelde K, McCarthy CL. Radiological findings of denosumab treatment for giant cell tumours of bone. Skeletal Radiol. 2020;49(9):1345–58.
Susarla SM, August M, Dewsnup N, Faquin WC, Kaban LB, Dodson TB. CD34 staining density predicts giant cell tumor clinical behavior. J Oral Maxillofac Surg. 2009;67(5):951–6.
Girolami I, Mancini I, Simoni A, Baldi GG, Simi L, Campanacci D, et al. Denosumab treated giant cell tumour of bone: a morphological, immunohistochemical and molecular analysis of a series. J Clin Pathol. 2016;69(3):240–7.
Yang Y-Q, Tan Y-Y, Wong R, Wenden A, Zhang L-K, Rabie ABM. The role of vascular endothelial growth factor in ossification. Int J Oral Sci. 2012;4(2):64–8.
Min J-K, Cho Y-L, Choi J-H, Kim Y, Kim JH, Yu YS, et al. Receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) increases vascular permeability: impaired permeability and angiogenesis in eNOS-deficient mice. Blood. 2007;109(4):1495–502.
van der Woude HJ, Verstraete KL, Hogendoorn PC, Taminiau AH, Hermans J, Bloem JL. Musculoskeletal tumors: does fast dynamic contrast-enhanced subtraction MR imaging contribute to the characterization? Radiology. 1998;208(3):821–8.
Lavini C, de Jonge MC, van de Sande MGH, Tak PP, Nederveen AJ, Maas M. Pixel-by-pixel analysis of DCE MRI curve patterns and an illustration of its application to the imaging of the musculoskeletal system. Magn Reson Imaging. 2007;25(5):604–12.
Van Den Berghe T, Verstraete KL, Lecouvet FE, Lejoly M, Dutoit J. Review of diffusion-weighted imaging and dynamic contrast-enhanced MRI for multiple myeloma and its precursors (monoclonal gammopathy of undetermined significance and smouldering myeloma). Skeletal Radiol. 2022;51(1):101–22.
Cuenod CA, Balvay D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging [Internet]. 2013;94(12):1187–204. https://doi.org/10.1016/j.diii.2013.10.010.
Huang G, Liu Z, Van Der Maaten L, Weinberger KQ. Densely connected convolutional networks. In: 2017 IEEE Conference on Computer Vision and Pattern Recognition (CVPR); 2017. p. 2261–9.
Amary F, Berisha F, Ye H, Gupta M, Gutteridge A, Baumhoer D, et al. H3F3A (Histone 3.3) G34W immunohistochemistry: a reliable marker defining benign and malignant giant cell tumor of bone. Am J Surg Pathol. 2017;41(8):1059–68.
Yang L, Zhang H, Zhang X, Tang Y, Wu Z, Wang Y, et al. Clinicopathologic and molecular features of denosumab-treated giant cell tumour of bone (GCTB): analysis of 21 cases. Ann Diagn Pathol [Internet]. 2022;57:151882. https://www.sciencedirect.com/science/article/pii/S1092913421001829
Acknowledgements
We would like to thank for technical support Sukru Karanfil, Ran Rumes and Lynn Supply from the pathology department. We would also like to thank Louis Deconinck for his help with the data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lejoly, M., Van Den Berghe, T., Creytens, D. et al. Diagnosis and monitoring denosumab therapy of giant cell tumors of bone: radiologic-pathologic correlation. Skeletal Radiol 53, 353–364 (2024). https://doi.org/10.1007/s00256-023-04403-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-023-04403-7