Abstract
Objective
To analyze the results of annual screening using whole-body magnetic resonance imaging (WBMRI) in patients with multiple hereditary exostoses (MHE) and enchondromatosis (EC), and estimate the risk for transformation to chondrosarcoma (CS) in these disorders.
Materials and methods
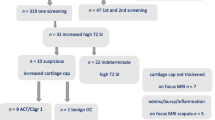
A total of 62 patients (57 with MHE and five with EC) screened during a mean follow-up period of 4.6 years (range, 1–10 years) using 253 WBMRIs (median four WBMRIs per patient, range, 1–10) were analyzed retrospectively. The time of WBMRIs was compared with dates for diagnosed CSs. A supplementary literature review was performed focusing on the risk of malignant transformation.
Results
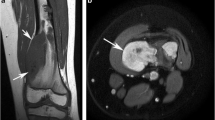
Ten patients had CS before being enrolled in the screening program, nine with MHE and one with EC. Three asymptomatic CSs were detected by screening; one in a patient with EC and two in patients with MHE, one of whom had CS previously. During the screening period, there was no occurrence of CS not detected by WBMRI in the study group.
Histopathologically, the CSs were predominantly grade 1 and were, except for in two patients, located at the truncus, proximal femur, and shoulder girdle.
Based on the current material and literature review, the risk of CS seems to be in the range of 2–3.7% for MHE and up to 50% for EC patients.
Conclusions
MRI may be used as a screening method detecting malignant transformation in MHE and EC patients, but the efficacy has to be confirmed in long-term follow-up studies including cost analysis.




Similar content being viewed by others
Change history
27 December 2019
Unfortunately in Volume 49, Issue 1 had been published online with an incorrect date (2001 instead of 2020).
References
Altay M, Bayrakci K, Yildiz Y, Erekul S, Saglik Y. Secondary chondrosarcoma in cartilage bone tumors: report of 32 patients. J Orthop Sci. 2007;12(5):415–23.
Black B, Dooley J, Pyper A, Reed M. Multiple hereditary exostoses. An epidemiologic study of an isolated community in Manitoba. Clin Orthop Relat Res. 1993;287(287):212–7.
Brien EW, Mirra JM, Luck JV. Jr. benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. II. Juxtacortical cartilage tumors. Skelet Radiol. 1999;28(1):1–20.
Clement ND, Ng CE, Porter DE. Shoulder exostoses in hereditary multiple exostoses: probability of surgery and malignant change. J Shoulder Elb Surg. 2011;20(2):290–4.
Czajka CM, DiCaprio MR. What is the proportion of patients with multiple hereditary exostoses who undergo malignant degeneration? Clin Orthop Relat Res. 2015;473(7):2355–61.
Gordon SL, Buchanan JR, Ladda RL. Hereditary multiple exostoses: report of a kindred. J Med Genet. 1981;18(6):428–30.
Crandall BF, Field LL, Sparkes RS, Spence MA. Hereditary multiple exostoses. Report of a family. Clin Orthop Relat Res. 1984;190(190):217–9.
Goud AL, de Lange J, Scholtes VA, Bulstra SK, Ham SJ. Pain, physical and social functioning, and quality of life in individuals with multiple hereditary exostoses in the Netherlands: a national cohort study. J Bone Joint Surg Am. 2012;94(11):1013–20.
Kivioja A, Ervasti H, Kinnunen J, Kaitila I, Wolf M, Bohling T. Chondrosarcoma in a family with multiple hereditary exostoses. J Bone Joint Surg Br. 2000;82(2):261–6.
Francannet C, Cohen-Tanugi A, Le Merrer M, Munnich A, Bonaventure J, Legeai-Mallet L. Genotype-phenotype correlation in hereditary multiple exostoses. J Med Genet. 2001;38(7):430–4.
Pedrini E, Jennes I, Tremosini M, et al. Genotype-phenotype correlation study in 529 patients with multiple hereditary exostoses: identification of "protective" and "risk" factors. J Bone Joint Surg Am. 2011;93(24):2294–302.
Pierz KA, Stieber JR, Kusumi K, Dormans JP. Hereditary multiple exostoses: one center’s experience and review of etiology. Clin Orthop Relat Res. 2002;401(401):49–59.
Porter DE, Lonie L, Fraser M, et al. Severity of disease and risk of malignant change in hereditary multiple exostoses. A genotype-phenotype study. J Bone Joint Surg Br. 2004;86(7):1041–6.
Schmale GA, Conrad EU 3rd, Raskind WH. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994;76(7):986–92.
Wicklund CL, Pauli RM, Johnston D, Hecht JT. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995;55(1):43–6.
Vanhoenacker FM, Van Hul W, Wuyts W, Willems PJ, De Schepper AM. Hereditary multiple exostoses: from genetics to clinical syndrome and complications. Eur J Radiol. 2001;40(3):208–17.
Tong K, Liu H, Wang X, et al. Osteochondroma: review of 431 patients from one medical institution in South China. J Bone Oncol. 2017;8:23–9.
Voutsinas S, Wynne-Davies R. The infrequency of malignant disease in diaphyseal aclasis and neurofibromatosis. J Med Genet. 1983;20(5):345–9.
Liu J, Hudkins PG, Swee RG, Unni KK. Bone sarcomas associated with Ollier’s disease. Cancer. 1987;59(7):1376–85.
Brien EW, Mirra JM, Kerr R. Benign and malignant cartilage tumors of bone and joint: their anatomic and theoretical basis with an emphasis on radiology, pathology and clinical biology. I The intramedullary cartilage tumors. Skeletal Radiol. 1997;26(6):325–53.
Verdegaal SH, Bovee JV, Pansuriya TC, et al. Incidence, predictive factors, and prognosis of chondrosarcoma in patients with Ollier disease and Maffucci syndrome: an international multicenter study of 161 patients. Oncologist. 2011;16(12):1771–9.
Hameetman L, Bovee JV, Taminiau AH, Kroon HM, Hogendoorn PC. Multiple osteochondromas: clinicopathological and genetic spectrum and suggestions for clinical management. Hered Cancer Clin Pract. 2004;2(4):161–73.
Gariani J, Westerland O, Natas S, Verma H, Cook G, Goh V. Comparison of whole-body magnetic resonance imaging (WBMRI) to whole-body computed tomography (WBCT) or (18)F-fluorodeoxyglucose positron emission tomography/CT ((18)F-FDG PET/CT) in patients with myeloma: systematic review of diagnostic performance. Crit Rev Oncol Hematol. 2018;124:66–72.
Sampath Kumar V, Tyrrell PN, Singh J, Gregory J, Cribb GL, Cool P. Surveillance of intramedullary cartilage tumours in long bones. Bone Joint J. 2016;98-B(11):1542–7.
Anupindi SA, Bedoya MA, Lindell RB, et al. Diagnostic performance of whole-body MRI as a tool for Cancer screening in children with genetic Cancer-predisposing conditions. AJR Am J Roentgenol. 2015;205(2):400–8.
Greer MC, Voss SD, States LJ. Pediatric cancer predisposition imaging: focus on whole-body MRI. Clin Cancer Res. 2017;23(11):e6–e13.
Saya S, Killick E, Thomas S, Taylor N, Bancroft EK, Rothwell J, et al. Baseline results from the UK SIGNIFY study: a whole-body MRI screening study in TP53 mutation carriers and matched controls. Familial Cancer. 2017;16(3):433–40.
Bernard SA, Murphey MD, Flemming DJ, Kransdorf MJ. Improved differentiation of benign osteochondromas from secondary chondrosarcomas with standardized measurement of cartilage cap at CT and MR imaging. Radiology. 2010;255(3):857–65.
Ahmed AR, Tan TS, Unni KK, Collins MS, Wenger DE, Sim FH. Secondary chondrosarcoma in osteochondroma: report of 107 patients. Clin Orthop Relat Res. 2003;(411):193–206 doi(411):193–206.
Sonne-Holm E, Wong C, Sonne-Holm S. Multiple cartilaginous exostoses and development of chondrosarcomas—a systematic review. Dan Med J. 2014;61(9):A4895.
Pansuriya TC, Kroon HM, Bovee JV. Enchondromatosis: insights on the different subtypes. Int J Clin Exp Pathol. 2010;3(6):557–69.
Wuisman PI, Jutte PC, Ozaki T. Secondary chondrosarcoma in osteochondromas. Medullary extension in 15 of 45 cases. Acta Orthop Scand. 1997;68(4):396–400.
Goud AL, Wuyts W, Bessems J, Bramer J, van der Woude HJ, Ham J. Intraosseous atypical chondroid tumor or chondrosarcoma grade 1 in patients with multiple osteochondromas. J Bone Joint Surg Am. 2015;97(1):24–31.
Hendel HW, Daugaard S, Kjaer A. Utility of planar bone scintigraphy to distinguish benign osteochondromas from malignant chondrosarcomas. Clin Nucl Med. 2002;27(9):622–4.
Subhawong TK, Winn A, Shemesh SS, Pretell-Mazzini J. F-18 FDG PET differentiation of benign from malignant chondroid neoplasms: a systematic review of the literature. Skelet Radiol. 2017;46(9):1233–9.
Staal HM, Dremmen MHG, Robben SGF, Witlox AMA, Rhijn LW. The use of whole-body MR imaging in children with HMO, an extended case study in two patients. Pediatr Ther. 2016;6:275.
Doyle LA. Sarcoma classification: an update based on the 2013 World Health Organization classification of tumors of soft tissue and bone. Cancer. 2014;120:1763–74.
The Skeletal Lesions Interobserver Correlation among Expert Diagnosticians (SLICED) Study Group. Reliability of histopathologic and radiologic grading of cartilaginous neoplasms in long bones. J Bone Joint Surg Am. 2007;89:2113–23.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was not externally funded and none of the authors has conflicts of interests to disclose. Patient consent and approval from The Central Denmark Region Committees on Health Research Ethics was waived due to the retrospective quality control design of the study. The data extract was approved by the Data Protection Agency in the Central Denmark Region (protocol number 1–16–02-114-18).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jurik, A.G., Jørgensen, P.H. & Mortensen, M.M. Whole-body MRI in assessing malignant transformation in multiple hereditary exostoses and enchondromatosis: audit results and literature review. Skeletal Radiol 49, 115–124 (2020). https://doi.org/10.1007/s00256-019-03268-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-019-03268-z