Abstract
Objective
To determine abdominal adipose tissue parameters on PET/CT in patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM) that may serve as predictors of progression of MGUS to MM. We hypothesized that patients with MM had higher abdominal adiposity and higher fat metabolic activity compared to patients with MGUS.
Materials and methods
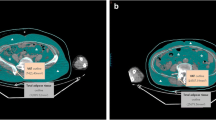
Our retrospective study was IRB approved and HIPAA compliant. The study group comprised 40 patients (mean age 64 ± 13 years) with MGUS and 32 patients (mean age 62 ± 10 years) with recently diagnosed MM (mean time since diagnosis of MM 3.0 ± 3.9 months) who had not undergone MM treatment. All patients underwent whole body FDG-PET/CT. Total abdominal adipose tissue (TAT), abdominal subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) cross sectional areas (CSA) (cm2) and metabolic activity (SUV) were assessed. Groups were compared using ANOVA. ROC curve analysis was performed to determine cutoff values for abdominal adipose tissue parameters to detect MM.
Results
Patients with recently diagnosed MM had higher TAT and SAT CSA (p ≤ 0.03) and higher fat metabolic activity (p < 0.01). VAT metabolic activity showed the highest sensitivity and specificity for identifying patients with MM (area under the curve 0.95 with cutoff value of >0.34, sensitivity 90.6 %, specificity 92.5 %, p < 0.0001).
Conclusions
Patients who were recently diagnosed with MM had higher abdominal fat CSA and higher fat metabolic activity compared to patients with MGUS. These parameters may serve as novel biomarkers of progression of MGUS to MM.



Similar content being viewed by others
References
Palumbo A, Anderson K. Multiple myeloma. N Engl J Med. 2011;364:1046–60.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30.
Riedel DA, Pottern LM. The epidemiology of multiple myeloma. Hematol Oncol Clin North Am. 1992;6:225–47.
Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7.
Kyle RA, Therneau TM, Rajkumar SV, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002;346:564–9.
Alexander DD, Mink PJ, Adami HO, et al. Multiple myeloma: a review of the epidemiologic literature. Int J Cancer. 2007;120 Suppl 12:40–61.
Blair CK, Cerhan JR, Folsom AR, Ross JA. Anthropometric characteristics and risk of multiple myeloma. Epidemiology. 2005;16:691–4.
Brown LM, Gridley G, Pottern LM, et al. Diet and nutrition as risk factors for multiple myeloma among blacks and whites in the United States. Cancer Causes Control. 2001;12:117–25.
Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y. Association of obesity and cancer risk in Canada. Am J Epidemiol. 2004;159:259–68.
Samanic C, Gridley G, Chow WH, Lubin J, Hoover RN, Fraumeni Jr JF. Obesity and cancer risk among white and black United States veterans. Cancer Causes Control. 2004;15:35–43.
Teras LR, Kitahara CM, Birmann BM, et al. Body size and multiple myeloma mortality: a pooled analysis of 20 prospective studies. Br J Haematol. 2014;166:667–76.
Christen T, Sheikine Y, Rocha VZ, et al. Increased glucose uptake in visceral versus subcutaneous adipose tissue revealed by PET imaging. JACC Cardiovasc Imaging. 2010;3:843–51.
Oliveira AL, Azevedo DC, Bredella MA, Stanley TL, Torriani M. Visceral and subcutaneous adipose tissue FDG uptake by PET/CT in metabolically healthy obese subjects. Obesity (Silver Spring). 2015;23:286–9.
Vongsuvanh R, George J, Qiao L, van der Poorten D. Visceral adiposity in gastrointestinal and hepatic carcinogenesis. Cancer Lett. 2012;330:1–10.
Cottet V, Vaysse C, Scherrer ML, et al. Fatty acid composition of adipose tissue and colorectal cancer: a case-control study. Am J Clin Nutr. 2015;101:192–201.
Dammacco F, Rubini G, Ferrari C, Vacca A, Racanelli V. (1)(8)F-FDG PET/CT: a review of diagnostic and prognostic features in multiple myeloma and related disorders. Clin Exp Med. 2015;15:1–18.
Ryo M, Kishida K, Nakamura T, Yoshizumi T, Funahashi T, Shimomura I. Clinical significance of visceral adiposity assessed by computed tomography: a Japanese perspective. World J Radiol. 2014;6:409–16.
Bredella MA, Torriani M, Ghomi RH, et al. Adiponectin is inversely associated with intramyocellular and intrahepatic lipids in obese premenopausal women. Obesity (Silver Spring). 2011;19:911–6.
Hyun YJ, Kim OY, Jang Y, et al. Evaluation of metabolic syndrome risk in Korean premenopausal women: not waist circumference but visceral fat. Circ J. 2008;72:1308–15.
Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–7.
Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond). 2007;31:500–6.
International Myeloma Working Group. Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol 2003;121:749–757
Durie BG, Waxman AD, D’Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002;43:1457–63.
Caldarella C, Treglia G, Isgro MA, Treglia I, Giordano A. The role of fluorine-18-fluorodeoxyglucose positron emission tomography in evaluating the response to treatment in patients with multiple myeloma. Int J Mol Imaging. 2012;2012:175803.
Lu YY, Chen JH, Lin WY, et al. FDG PET or PET/CT for detecting intramedullary and extramedullary lesions in multiple Myeloma: a systematic review and meta-analysis. Clin Nucl Med. 2012;37:833–7.
Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–74.
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–38.
Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52:1779–85.
Bao Y, Giovannucci EL, Kraft P, et al. A prospective study of plasma adiponectin and pancreatic cancer risk in five US cohorts. J Natl Cancer Inst. 2012;105:95–103.
Miyoshi Y, Funahashi T, Kihara S, et al. Association of serum adiponectin levels with breast cancer risk. Clin Cancer Res. 2003;9:5699–704.
Hofmann JN, Liao LM, Pollak MN, et al. A prospective study of circulating adipokine levels and risk of multiple myeloma. Blood. 2012;120:4418–20.
Fowler JA, Lwin ST, Drake MT, et al. Host-derived adiponectin is tumor-suppressive and a novel therapeutic target for multiple myeloma and the associated bone disease. Blood. 2011;118:5872–82.
Klein B, Tarte K, Jourdan M, et al. Survival and proliferation factors of normal and malignant plasma cells. Int J Hematol. 2003;78:106–13.
Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83.
Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–5.
Doucette CR, Horowitz MC, Berry R, et al. A high fat diet increases bone marrow adipose tissue (MAT) but does not alter trabecular or cortical bone mass in C57BL/6J mice. J Cell Physiol. 2015;230:2032–7.
Lwin ST, Olechnowicz SW, Fowler JA, Edwards CM. Diet-induced obesity promotes a myeloma-like condition in vivo. Leukemia. 2015;29:507–10.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
None
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was waived for individual participants included in the study. The study was approved by the local Institutional Review Board (IRB) and HIPAA compliant.
IRB approval
The study was IRB approved.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 3353 kb)
Rights and permissions
About this article
Cite this article
Veld, J., O’Donnell, E.K., Reagan, M.R. et al. Abdominal adipose tissue in MGUS and multiple myeloma. Skeletal Radiol 45, 1277–1283 (2016). https://doi.org/10.1007/s00256-016-2425-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00256-016-2425-4