Abstract
β-1,6-Glucan plays a crucial role in fungal cell walls by linking the outer layer of mannoproteins and the inner layer of β-1,3-glucan, contributing significantly to the maintenance of cell wall rigidity. Therefore, the hydrolysis of β-1,6-glucan by β-1,6-glucanase directly leads to the disintegration of the fungal cell wall. Here, a novel β-1,6-glucanase FlGlu30 was identified from the endophytic Flavobacterium sp. NAU1659 and heterologously expressed in Escherichia coli BL21 (DE3). The optimal reaction conditions of purified FlGlu30 were 50℃ and pH 6.0, resulting in a specific activity of 173.1 U/mg using pustulan as the substrate. The hydrolyzed products of FlGlu30 to pustulan were mainly gentianose within 1 h of reaction. With the extension of reaction time, gentianose was gradually hydrolyzed to glucose, indicating that FlGlu30 is an endo-β-1,6-glucanase. The germination of Magnaporthe oryzae Guy11 spores could not be inhibited by FlGlu30, but the appressorium formation of spores was completely inhibited under the concentration of 250.0 U/mL FlGlu30. The disruptions of cell wall and accumulation of intracellular reactive oxide species (ROS) were observed in FlGlu30-treated M. oryzae Guy11 cells, suggesting the significant importance of β-1,6-glucan as a potential antifungal target and the potential application of FlGlu30.
Key points
• β-1,6-Glucan is a key component maintaining the rigid structure of fungal cell wall.
• β-1,6-Glucanase is an antifungal protein with significant potential applications.
• FlGlu30 is the first reported β-1, 6-glucanase derived from Flavobacterium.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The fungus Magnaporthe oryzae is the cause of rice blast, a highly destructive disease that severely impacts rice cultivation worldwide (Jin et al. 2024). Currently, efforts to control and manage rice blast include the development of resistant rice varieties, cultural practices and fungicide applications (Asibi et al. 2019). The breeding of resistant rice varieties involves a lengthy and resource-intensive process; therefore, chemical agents remain the primary means of controlling rice blast. However, with increasing concern about the environmental problems caused by chemical agents, researchers have begun to use more environmentally friendly biological control methods as alternatives (Haas and Defago 2005; Ye et al. 2020a,b).
Fungal cell walls are vital for cell integrity, as they provide mechanical protection from environmental stress (Gow et al. 2017). The fungal cell walls contain approximately 50–60% glucans, with β-1,6-glucan accounting for around 10% of the total glucan content (Ruiz-Herrera and Ortiz-Castellanos 2019). Serving as a cross-linking agent, β-1,6-glucan enhances the rigidity of the cell wall structure (Ye et al. 2023). Therefore, the degradation of β-1,6-glucan by β-1,6-glucanase can diminish the mechanical resilience of the cell walls, ultimately resulting in the lysis of fungal cells (Li et al. 2019; Ye et al. 2023). Additionally, these enzymes may also be involved in regulating fungal diseases (Fayad et al. 2001; Yamamoto et al. 1974).
β-1,6-Glucanase (EC 3.2.1.75) is an enzyme that specifically targets and hydrolyzes β-1,6-glycosidic bonds present in various glucans (Plakys et al. 2024; Wang et al. 2017). β-1,6-Glucanases derived from eukaryotic or prokaryotic are classified into glycoside hydrolase (GH) families 5 and 30 in the CAZy database (http://www.cazy.org/) based on their amino acid sequences (Wang et al. 2017). The GH30 family of β-1,6-glucanases utilizes an acid–base mechanism to hydrolyze β-glycoside bonds (Rezaie et al. 2018). This mechanism necessitates the presence of a minimum of two amino acid residues in the enzyme’s active site, with one serving as an acid or base and the other functioning as a nucleophile (Park et al. 2000). These enzymes belong to the glycosyl hydrolase A family, characterized by their three-dimensional triosephosphate isomerase barrel structure. Typically, their active site contains two glutamic acid residues, and the enzymes primarily target substrates within the cell wall structures of filamentous fungi and yeasts (Rast et al. 2003). In addition, substrates of β-1,6-glucanases can also be found as secretory or storage polysaccharides, such as pustulan (β-1,6-glucan) and laminarin (β-1,3–1,6-glucan), in certain fungi and lichens (Pradeep and Edison 2022; Tupe et al. 2022).
β-1,6-Glucanases derived from the GH5 family have hardly been reported for antifungal properties. However, researchers have found that β-1,6-glucanase VfGlu1 derived from the GH5 family plays a key role in the infection of Agaricus bisporus by Verticillium fungicola (Amey et al. 2003). In addition, it has been reported that β-1,6-glucanases (EC 3.2.1.75) of some fungi are important for fungal mycoparasitism and probably cell wall cycling (Aspeborg et al. 2012). Therefore, we speculate that the β-1,6-glucanase sourced from the GH5 family may possess antifungal activity, but this requires more direct experimental verification.
In this study, the gene encoding β-1,6-glucanase FlGlu30, belongs to GH30 family, was cloned from Flavobacterium sp. NAU1659 and heterologously expressed in Escherichia coli BL21(DE3). The recombinant β-1,6-glucanase FlGlu30 was purified using Ni2+-NTA and exhibited maximal activity with pustulan. Consequently, the enzymatic properties of FlGlu30 and its antifungal effects on M. oryzae Guy11 were investigated, respectively. Finally, the integrity of cell wall and accumulation of reactive oxygen species (ROS) in FlGlu30-treated Guy11 cells were analyzed to understand the antifungal mechanisms of FlGlu30. This is the first report of a β-1,6-glucanase from endophytic bacteria, laying the theoretical foundation for the application of β-1,6-glucanase in the biological control of plant pathogenic fungi.
Materials and methods
Strains, plasmids and reagents
Peptone and yeast extract were purchased from Oxoid Co. Ltd. (Beijing, China). All molecular biology reagents were purchased from TaKaRa Co., Ltd. (Otsu, Japan). Pustulan (Elicityl, Crolles, France) were purchased from Shanghai ZZBIO Co., Ltd. (Shanghai, China). Laminarin (from Laminaria digitata), pachyman, xylan, and carboxymethyl cellulose-sodium salt (CMC) were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Flavobacterium sp. NAU1659, which was isolated from cucumbers’ rhizosphere soil and maintained in our lab, and E. coli BL21(DE3), purchased from Takara Bio Company (Kusatsu, Japan), were cultured in Luria–Bertani (LB) medium containing 10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl at 30 ℃ and 37 ℃, respectively. M. oryzae strain Guy11 (ATCC 201236) was cultured in complete medium (CM) with or without 1.5% agar at 25 ℃ for 3–5 days. The plasmid pET-29a ( +), which was used for cloning and expressing of β-1,6-glucanase FlGlu30 (GenBank accession number: PP690410), was obtained from Vazyme (Nanjing, China). The genomic DNA of strain NAU1659 was extracted following a previous report (Ye et al. 2022).
Gene cloning, heterologous expression and purification of β-1,6-glucanase FlGlu30
The signal peptide of FlGlu30 was predicted using the Signal-3L 3.0 server (http://www.csbio.sjtu.edu.cn/bioinf/Signal-3L/). The coding regions corresponding to the gene, excluding the signal peptide, were amplified from the extracted DNA using the primer pairs FlGlu30-F (5′-TAAGAAGGAGATATACATATGTCAAAAA ATGTTACTGCCAATTC-3′) and FlGlu30-R (5′-GTGGTGGTGGTGGTGGT GCTCGAGTTACCAACGAAAGTAGCAACTGC-3′). The products were ligated to the NdeI/XhoI digested expression vector pET-29a, purchased from Takara Bio Company (Kusatsu, Japan), to generate the recombinant plasmid pET-29a-FlGlu30. The ClonExpress II/One Step Cloning Kit (Vazyme, Nanjing, China) was employed for this purpose.
The recombinant plasmid was then introduced into E. coli BL21(DE3), and the transformed cells were screened and verified through DNA sequencing. Positive transformants were selected and cultured at 37 °C in LB medium supplemented with 100 μg/mL kanamycin until the optical density (OD600) reached 0.6–0.8. Subsequently, the culture medium was supplemented with isopropyl-β-D-1- thiogalactopyranoside (IPTG) at a final concentration of 0.2 mM to induce protein expression and further incubated at 16 °C for 20 h.
The cells harvested from the induced culture were suspended in 50 mM PBS buffer (pH 6.0) and disrupted by ultrasonication (Sonicator 201 M, Kubota, Osaka, Japan). The mixture of disrupted cells was centrifuged at 12,000 rpm for 10 min at 4 °C to separate into soluble and insoluble fractions. The recombinant fusion proteins, tagged with a C-terminal 6His-tag, were purified using Ni2+-nitrilotriacetic acid (Transgen, Beijing, China) resin following the manufacturer’s instructions. Then, the purified FlGlu30 protein was collected and dialyzed overnight at 4 °C against 50 mM PBS buffer (pH 6.0) to remove imidazole. Protein purity was determined using SDS-PAGE, and protein concentration was assessed using the Bradford method (Bradford 1976).
Enzyme characteristics of β-1,6-glucanase FlGlu30
The activity of the purified recombinant enzyme was assessed by measuring the release of reducing sugars from a pustulan solution (5 mg/mL). The reaction mixture contained 60 μL of the pustulan and 5 μg/mL of purified FlGlu30 was incubated at 50 °C for 10 min. Subsequently, an equal volume of the 3,5-dinitrosalicylic acid reagent was added to the reaction and incubated at 100 °C for 10 min (Gusakov et al. 2011). One unit of enzyme activity was defined as the quantity of enzyme needed to liberate reducing sugars equivalent to 1 μmol of glucose per minute under the specified testing conditions.
The characteristics of β-1,6-glucanase FlGlu30 were analyzed using 0.5% pustulan as the substrate. The optimal temperature for β-1,6-glucanase FlGlu30 activity was determined within a range of 20–80 °C, with intervals of 10 °C, in a 50 mM PBS buffer at pH 6.0. To assess the thermal stability of the enzyme, the enzyme solution was incubated at different temperatures (4 °C, 20 °C, 30 °C, 40 °C, 50 °C, 60 °C, 70 °C and 80 °C) for 1 h, 2 h, 4 h and 8 h. After the respective incubation periods, 0.5% pustulan was added to the enzyme solution at 50 °C for a 10 min. The residual hydrolytic activity of the enzyme was measured. The initial enzyme activity of FlGlu30 that was not subjected to incubation at different temperatures was used as a reference to calculate the relative enzyme activity after incubation. FlGlu30 exhibited the most favorable pH at 50 °C by evaluating its performance across different buffers: 50 mM sodium acetate buffer at pH 4.0–6.0, 50 mM Tris–HCl buffer at pH 6.0–9.0, 50 mM PBS buffer at pH 6.0–8.0 and 50 mM glycine–NaOH buffer at pH 9.0–11.0. To evaluate its pH stability, the activity of FlGlu30 was measured under standard conditions after incubating for 24 h at 4 °C in the aforementioned buffers without substrate.
The enzymatic activity of FlGlu30 was evaluated for its susceptibility to potential inhibitors or activators. This was accomplished by introducing a concentration of 1 mM of various metal salts (Ni2+, Ba2+, Mg2+, Zn2+, Fe2+, Co2+, Na+, Al3+, Ca2+, Mn2+, Cu2+, K+, Cr3+) and other chemical agents at various concentrations (methanol, ethanol, isopropanol, acetone, acetonitrile, ethylene diamine tetraacetic acid (EDTA), dimethyl sulfoxide (DMSO), Tween 80, Triton X-100, β-mercaptoethanol (β-ME), urea, dithiothreitol (DTT), phenylmethanesulfonyl fluoride (PMSF)) into the reaction mixture. Following 1 h of incubation at 40 °C, the residual activity was measured at 50 °C for 10 min after supplement with 0.5% pustulan.
To determine the substrate specificity of FlGlu30, the enzyme activity was measured in 50 mM PBS buffer (pH 6.0) containing 5 mg/mL of each substrate, including pustulan (β-1,6-glucan), yeast glucan (β-1,3–1,6-glucan), laminarin (β-1,3–1,6-glucan), pachyman (β-1,3-glucan), xylan (β-1,4-glucan), and CMC (β-1,4-glucan). The reaction suppled with inactive FlGlu30 used as the control. In addition, the different concentrations of pustulan ranging from 1 to 10 mg/mL mixed with purified FlGlu30 under the optimal conditions for 10 min were used to measure the kinetic constants of FlGlu30. The kinetic rate constants, Km and Vmax, were obtained by examining the data using a Lineweaver–Burk plot (Dowd and Riggs 1965).
Analysis of the hydrolysis products
Purified FlGlu30 (10 μg) was added to reaction mixture containing 0.5% pustulan and then incubated at 50 ℃ for different time (1 min, 5 min, 10 min, 30 min, 1 h, 2 h, 4 h, 8 h, 12 h, 16 h, and 24 h) in 50 mM PBS buffer (pH 6.0). After incubation, the reaction mixtures were promptly boiled at 100 ℃ for 10 min and centrifuged at 12,000 rpm for 3 min. Subsequently, the released oligosaccharides were analyzed using thin-layer chromatography (TLC). The samples were spotted onto a TLC plate, developed in n-butanol/acetic acid/water (2:1:1, v/v/v) as a solvent, and then sprayed with sulfuric acid/methanol (1:1). The products were displayed after heating the sheet at 95 ℃ in an oven for 5 min.
Inhibitory effect of β-1,6-glucanase FlGlu30 on spore germination of Guy11
For spore production, the mycelia were cultured on a corn agar medium (SDC) containing 100 g of rice straw, 40 g of corn powder, and 15 g of agar dissolved in 1 L of deionized water. Subsequently, the plates containing mycelia were incubated at a temperature of 28 °C for 3 days in a dark environment. Then, the aerial hyphae were scraped from the culture, and the plates were subjected to continuous illumination under light for an additional 3 days (Qi et al. 2016).
The conidia were washed with ddH2O, and passed through three-layer lens paper to filter out hyphae. Then, the conidia were collected by centrifugation at 5000 rpm for 5 min and re-suspended in 200 μL of 50 mM PBS buffer (pH 6.0). Purified FlGlu30 was sterilized though a 0.22 μm pore-size filter. Finally, the conidia were mixed with different concentration of purified FlGlu30 and spotted on a cover glass (12542B, Fisherbrand, ThermoFisher, Waltham, MA, USA) to calculate the germination rate of conidia and appressorium formation under a microscopic (CX23, Olympus, Tokyo, Japan). The final concentration of conidia in mixtures was 5 × 104 cells/mL using a hemocytometer (Wang et al. 2021). The heat-inactivated FlGlu30 was used as control.
Analysis of cell membrane integrity, reactive oxygen species (ROS) and chitin content of FlGlu30-treated Guy11 cells
The detections of cell membrane integrity, ROS and chitin content of FlGlu30 treated Guy11 cells were performed following previous description with minor modifications (Ye et al. 2023). The final concentration of 5 × 104 conidia/mL Guy11 cells were incubated with 160 U/mL FlGlu30 at 28 ℃ for 1.0 and 3.0 h, respectively. The cells were washed and re-suspended in a 50 mM PBS buffer at pH of 6.0 after FlGlu30 treatment. Subsequently, 5.0 μM propidium iodide (PI) and 10 mg/mL of calcofluor white (CFW) were added and incubated for another 20 min and 5 min in darkness, respectively. Moreover, FlGlu30-treated conidia were mixed with 50 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) for a duration of 20 min at room temperature to analysis ROS content. The spores were washed with PBS buffer prior to the mentioned staining steps to ensure cleanliness, and after staining, any residual dye was washed off using the same buffer solution. All images were captured and visualized using a confocal laser scanning microscopy (CLSM) (Leica TCS SP8, Wetzlar, Germany).
Statistical analysis
All analyses and measurements were performed in triplicate. The values are represented by the mean ± standard deviation (SD). Single-factor analysis of variance (one-way ANOVA) and Duncan’s multiple range test were carried out using SPSS statistics software version 22.0 (IBM Corporation, Armonk, NY, USA) to evaluate the significant differences (P ≤ 0.05) between the different treatments.
Results
Identification of the β-1,6-glucanase FlGlu30 from Flavobacterium sp. NAU1659
A novel β-1,6-glucanase FlGlu30 from Flavobacterium sp. NAU1659 was identified by searching nucleotide sequence in the NCBI database. The complete coding sequence of FlGlu30 consists of 1425 base pairs that encode for 474 amino acids. The protein has a calculated molecular weight of 52.2 kDa and a pI of 7.6. Analysis of the Signal-3L 3.0 server revealed the presence of a signal peptide (MKNINKKLQILVLLPLIAM QLNCGS) at the N-terminal region of the protein.
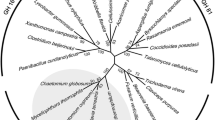
As shown in Fig. 1, FlGlu30 was aligned with other β-1,6-glucanases from the GH5 and GH30 family including fungal and bacterial enzymes. The phylogenetic analysis of the β-1,6-glucanases amino acid sequence showed that FlGlu30 is a member of the GH30 family and closely related to the bacterial β-1,6-glucanases. In the list provided, the proteins identified from Saccharophagus degradans (ABD82251, 49.3%), Bacillus mesophilum (KAB2330047.1, 39.8%), and Paenibacillus polymyxa (WP016819904.1, 38.9%) exhibited the highest homology to FlGlu30. The similarity between other β-1,6-glucanase in the GH30 family and FlGlu30 ranges from 27.2 to 36.4%.
Phylogenetic analysis of β-1,6-glucanase from various sources. The distances were determined and the phylogenetic tree was constructed using the neighbor-joining algorithm based on the amino acid sequence alignment in MEGA7 (Kumar et al. 2016). Bootstrap values based on 1000 replications are listed as percentages at branch points. Bar, 0.1 substitutions per amino acid position. GH30 and GH5 represent glycoside hydrolase family 30 and 5, respectively. β-1,6-Glucanase FlGlu30 from Flavobacterium sp. NAU1659 was represented in bold black font
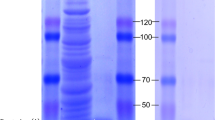
The purification process and harvest efficiency of recombinant FlGlu30 protein were showed in Table 1. Through a two-step purification process, FlGlu30 was purified 20.48-fold, resulting in a 34.64% recovery and a specific activity of 173.12 U/mg using pustulan as a substrate. After that, SDS-PAGE was conducted to determine the apparent molecular mass of the recombinant protein. The purified FlGlu30 protein exhibited a cleared band on SDS-PAGE, with an approximate molecular weight of around 50 kDa (Fig. 2).
SDS-PAGE analysis of the recombinant FlGlu30 protein. Purified FlGlu30 protein was loaded onto a 12.0% Tris–glycine SDS-PAGE gel and were stained with Coomassie brilliant blue R-250. M: protein low molecular weight marker; lane 1: The cell disruption supernatant of E. coli BL21(DE3) containing pET-29a-FlGlu30 recombinant plasmid; lane 2: The cell disruption precipitate of E. coli BL21(DE3) containing pET-29a-FlGlu30 recombinant plasmid; lane 3: flow-through of cell disruption supernatant after binding to the Ni2+ column; lane 4: the eluent solution after the Ni2+ column washed with 20 mM Tris–HCl buffer; lane 5–8: different purities of FlGlu30 protein after washing with 20 mM Tris–HCl buffer containing 50, 100, 200 and 300 mM imidazolium, respectively
Effects of pH and temperature on recombinant FlGlu30 activity and stability
The enzymatic properties of FlGlu30 were determined utilizing pustulan as the substrate. The results showed that purified FlGlu30 exhibited clear optimum pH and temperature were 6.0 and 50 °C, respectively (Fig. 3a and c). Moreover, FlGlu30 exhibited excellent stability at 50 ℃, retaining over 80% of its activity after 1 h. About more than 50% of the enzyme activity was still detected after 8 h of incubation under below 30 ℃ (Fig. 3b). However, a notable decline in enzymatic stability was observed after 1 h when the temperature exceeded 60 ℃, indicating β-1,6-glucanase FlGlu30 could be classified as a mesophilic enzyme. The purified FlGlu30 demonstrated a high level of activity within the pH range of 5.0 to 6.0, but showed minimal activity at pH values above 7.0 or below 5.0 (Fig. 3c). The enzyme retained more than 70% of its initial activity at pH 5.0 to 7.0 after incubation for 24 h. However, its stability decreased significantly at pH values below 5.0 or above 7.0 (Fig. 3d).
Effects of temperature and pH on the activity and stability of FlGlu30. (a) Determination of the optimal temperature of FlGlu30. Activity was measured in 50 mM PBS buffer (pH 6.0) at 20–70 ℃ for 10 min. (b) Thermostability of purified FlGlu30. The residual activity was measured under optimal conditions after incubation of the enzyme at the indicated temperatures for 1–8 h. (c) Determination of the optimal pH of FlGlu30. Assays were carried out with 5 mg/mL pustulan as substrate at 50 ℃ for 10 min in buffers with varying pH (pH 3.0–11.0). (d) pH stability of purified FlGlu30. The residual enzyme activity was measured under optimal conditions after incubation of the purified enzyme in buffers with various pH values at 4 ℃ for 24 h
Effect of metal ions and chemical agents on the enzymatic activity of FlGlu30
To analyze the effect of different metal ions or chemical reagents on FlGlu30 activity, the residual activities of the enzymes were measured following 1 h of incubation with 1 mM concentrations of various metal ions or chemical reagents, respectively. The results showed that K+ and Na+ had almost no effect on β-1,6-glucanase activity of FlGlu30 at the tested concentration (Table 2). Mg2+ and Mn2+ were found to have a slight stimulating effect on FlGlu30 activity, resulting in approximately 20% increase. However, the activity of FlGlu30 was significantly inhibited by Fe3+ and Zn2+. Additionally, it was observed that high concentrations of EDTA (10 mM), ethanol, isopropanol, acetone and acetonitrile completely inhibited the glucosidase activity of FlGlu30. Methanol and PMSF treatment resulted in approximately a 60% decrease in FlGlu30 activity. By contrast, Tween 80 and β-ME slightly improved the β-1,6-glucanase activity, whereas EDTA (1 mM), DMSO, Triton X-100, urea, and DTT had no significant effect.
Substrate specificity and analysis of the hydrolysis products
After conducting tests on polysaccharide substrates with different linkages, it was discovered that FlGlu30 enzyme effectively cleaved and liberated soluble glucose from pustulan. However, FlGlu30 showed no hydrolytic activity towards laminarin, which also contains β-1,6-glycosidic linkages, indicating potential substrate selectivity of the enzyme. Additionally, FlGlu30 exhibited no hydrolytic activity towards polysaccharides containing β-1,3 or β-1,4-glycosidic linkages, such as pachyman, xylan, and cellulose. Under the optimal conditions, the Michaelis–Menten constant (Km) and maximum reaction rate (Vmax) were found to be 4.2 mg·mL−1 and 318.1 μmol·min−1·mg−1, respectively. The above results suggested that pustulan, which solely contains β-1,6-glycosidic linkages, was the optimal substrate for FlGlu30. Therefore, we have confirmed that FlGlu30 functions as a β-1,6-glucanase.
Subsequently, the hydrolytic properties of FlGlu30 were investigated using pustulan (0.5%, w/v) as substrate by utilizing thin-layer chromatography (TLC). The results showed that after incubation of FlGlu30 with the pustulan for 1 min, the main product is gentianose, but there were also small amounts of oligosaccharides with chain lengths ≥ 4 generated. Throughout the entire reaction process, the content of gentianose initially increased and then decreased, ultimately being hydrolyzed by FlGlu30 into monosaccharides (Fig. 4). Therefore, we speculated that FlGlu30 was an endo-β-1,6-glucanase.
Effects of FlGlu30 on spores’ germination and appressorium formation
The inhibitory effect of FlGlu30 on M. oryzae Guy11 was determined by measuring the rate of spores’ germination and appressorium formation. The results showed that FlGlu30 could not inhibit the germination of spores, but could significantly inhibit the formation of appressorium (Fig. 5). Following an 8-h treatment with 200.0 U/mL FlGlu30, the appressorium formation rate of conidia was decreased significantly from 97.6% in control group to 17.3% in treatment group (Fig. 5b). Moreover, almost all spores’ germination were observed within 2 h after treatment with higher concentration of FlGlu30 (500.0 U/mL) (data not shown), suggesting that the low hydrolysis efficiency of FlGlu30 on the fungal cell wall may not inhibit the germination of spores. However, these findings highlight the potential significance of β-1,6-glucan in the fungal cell wall as a key target for impeding fungal growth.
Effects of FlGlu30 on the formation of germ tubes and appressoria. β-1,6-Glucanase FlGlu30 was added to the spores’ suspension for determine the effects of the enzyme on fungal germination (a). The number of germinated spores and appressorium formation was counted after treatment with FlGlu30 for different times (b). Treatments with heat-inactivated FlGlu30 were used as the control
Treatment with FlGlu30 resulted in the disruption of the cell membrane integrity in Guy11 cells
In order to gain further insights into the mechanisms underlying FlGlu30’s ability to inhibit appressorium formation, this study focused on evaluating the integrity of cell membranes, ROS accumulation, and chitin distribution within the cells. The intact cell membrane of a living cell is selectively permeable (McElhaney 1975). Therefore, propidium iodide (PI) cannot normally pass through the cell membrane and bind to DNA in the cells, however, it can enter cells with damaged cell membranes (Ye et al. 2023). By binding to the DNA of necrotic cell, propidium iodide (PI) exhibited red fluorescence under the fluorescence field, allowing us to identify impaired cell membranes based on the fluorescent color. Here, the results showed that after 1 h of treatment with FlGlu30, the mycelial cells showed red bright spots under the fluorescence field, indicating that PI entered the fungal cells and bound with DNA (Fig. 6). In addition, with the prolongation of treatment time, both of the proportion of mycelia containing fluorescent and the fluorescence intensity of hyphae were increased, indicating that hydrolysis of the fungal cell wall by FlGlu30 caused damage to Guy11 cells’ membranes (Fig. 6).
Treatment with FlGlu30 induced the burst of intracellular reactive oxygen species (ROS) in the cells
The induction of cell wall synthesis and regulation of cell wall integrity (CWI) in fungi are associated with the production of ROS (Fuchs and Mylonakis 2009; Yu et al. 2016). Upon entering the cell, H2DCFDA is oxidized by intracellular ROS, resulting in the generation of the fluorescent chromophore. So, H2DCFDA was often used to analyze the accumulation of intracellular ROS under environmental stress. The results showed that compared with the control group, intracellular ROS began to accumulate after FlGlu30 treatment for 1 h (Fig. 7). With the increase of treatment time, a small amount of ROS accumulated in the cells of control group after 3 h, but its content was significantly lower than that in FlGlu30 treated cells, indicating the hydrolysis stress of FlGlu30 on the fungal cell wall induced the burst of ROS (Fig. 7).
The pathway of cell wall integrity (CWI) was activated in FlGlu30-treated cells
The cell wall plays a vital role in preserving cell shape and safeguarding cells against harsh environmental conditions (Bermejo et al. 2008). When a component of the fungal cell wall is disrupted, it often triggers the fungal CWI pathway to increase the synthesis of other components to compensate for the damage caused by the absence of that component (Lu et al. 2023). Here, the CFW dye, which can specifically bind to chitin components in the fungal cell wall, is used to detect changes in the distribution of chitin in the cell wall after FlGlu30 treatment of M. oryzae cells. The results revealed that, in contrast to the uniform distribution of chitin in the cell wall of the control group, the cell wall of M. oryzae Guy11 spores exhibited an uneven distribution of chitin after being treated with FlGlu30 (Fig. 8). The same results were also reported in the Fusarium oxysporum cells treated with β-1,6-glucanase GluM (Ye et al. 2023).
Discussion
Most of the reported β-1,6-glucanases are derived from fungi, where they play important roles in fungal cell wall remodeling and inhibition of the growth of other fungi. For example, three β-1,6-glucanases, BGN16.1, BGN16.2, and BGN16.3, have been identified in the antagonistic fungus Trichoderma harzianum (de la Cruz and Llobell 1999; de la Cruz et al. 1995). The most active β-1,6-glucanase derived from fungi was GH30A, which sourced from Coprinopsis cinerea ATCC56838, with a hydrolytic activity of 776.5 U/mg towards 1% pustulan (Liu et al. 2020). Furthermore, an increasing number of β-1,6-glucanases sourced from bacteria are being reported. Such as, the β-1,6-glucanase GluM from Corallococcus sp. EGB exhibited high hydrolytic activity of up to 24,000 U/mg towards yeast glucan (Li et al. 2019). Researchers have found that this enzyme plays a crucial role in the predation of M. oryzae and F. oxysporum by the myxobacteria strain EGB (Li et al. 2019; Ye et al. 2023). Flavobacterium is an important plant rhizosphere microorganism capable of secreting abundant extracellular glycoside hydrolases to degrade organic macromolecules in the plant rhizosphere (Enisoglu-Atalay et al. 2018). However, no β-1,6-glucanase sourced from microorganisms belonging to the Flavobacterium genus has been reported. Here, the enzyme characteristics and antifungal properties of the β-1,6-glucanase FlGlu30 were studied and compared with the other reported β-1,6-glucanases. The findings enriched the repository of β-1,6-glucanases and lay the foundation for elucidating the potential ecological functions of Flavobacterium in the plant rhizosphere.
By utilizing fungal cell wall-degrading enzymes (CWDEs), such as chitinases, β-D-glucanases, chitosanases and proteases, bacteria are believed to exert their antifungal effects (Gutierrez-Gongora and Geddes-McAlister 2021; Takashima et al. 2023; Tue et al. 2024; Wang et al. 2021). These enzymes play a crucial role in the degradation of the structural components of fungal cell wall, effectively impeding the growth and proliferation of fungi (Ghasemi et al. 2020; Gutierrez-Gongora and Geddes-McAlister 2021). Furthermore, reducing the formation of appressoria on spores could significantly diminish the infections of rice by these spores, consequently lowering the incidence of rice blast disease (Huang et al. 2022; Shahriar et al. 2020). FlGlu30, possessing endo-β-1,6-glucanase activity, has been shown in antifungal assays to display antagonistic effects against fungus M. oryzae.
Intracellular ROS play a critical role in activities of cellular life (Ye et al. 2023). Low concentrations of ROS can serve as intercellular messengers for many activities; however, a burst of ROS can lead to apoptosis or cell death (Cadenas and Davies 2000). Ye et al. (2023) reported that the β-1,6-glucanase GluM from the Corallococcus sp. EGB hydrolyzed the cell wall of F. oxysporum, leading to the burst of intracellular ROS and subsequent apoptosis of fungal cells.
Here, the purified FlGlu30 enzyme has demonstrated promising efficacy in inhibiting the formation of appressoria by the Guy11 strain. The fungistatic effect of FlGlu30, as revealed by staining with PI, H2DCFDA and CFW, can be attributed, at least in part, to its capacity for hydrolyzing the cell walls of M. oryzae Guy11 cells. Organisms utilize multiple signaling pathways to react to extracellular stimuli and uphold their cell wall integrity in response to changes in their environment (Fuchs and Mylonakis 2009). These pathways include processes such as ROS generation and cell wall remodeling. Therefore, the disruption of cell membrane integrity, accumulation of intracellular ROS and the alterations in chitin distribution in the cell wall induced by FlGlu30 treatment on M. oryzae Guy11 suggesting that β-1,6-glucan could serve as a key target for the biological control of pathogenic fungi.
In conclusion, a novel β-1,6-glucanase FlGlu30 gene was cloned from genome of Flavobacterium sp. NAU1659 and heterologously expressed in E. coli BL21(DE3). The optimum reaction temperature and pH of FlGlu30 were 50℃ and 6.0, respectively. Under the optimum reaction conditions, the specific enzyme activity of FlGlu30 with pustulan as substrate was 173.1 U/mL. The hydrolysis products, primarily consisting of gentianose, were detected at the beginning of the reaction, and with prolonged reaction time, pustulan was ultimately hydrolyzed into monosaccharides, indicating that FlGlu30 is an endo-β-1,6-glucanase. The appressorium formation of spores of M. oryzae Guy11 was completely inhibited by 250.0 U/mL FlGlu30. PI and H2DCFDA staining showed that hydrolysis of Guy11 cell wall by FlGlu30 resulted in the disruptions of cell membrane and accumulation of intracellular ROS. This study provides a theoretical basis and genetic resources for the application of β-1,6-glucanase in the biological control of plant pathogenic fungi.
Data availability
The data that support the results of this research are available from the corresponding author.
References
Amey RC, Mills PR, Bailey A, Foster GD (2003) Investigating the role of a Verticillium fungicola β-1,6-glucanase during infection of Agaricus bisporus using targeted gene disruption. Fungal Genet Biol 39(3):264–275
Asibi AE, Chai Q, Coulter JA (2019) Rice blast: a disease with implications for global food security. Agronomy 9(8):451
Aspeborg H, Coutinho PM, Wang Y, Brumer H, Henrissat B (2012) Evolution, substrate specificity and subfamily classification of glycoside hydrolase family 5 (GH5). BMC Evol Biol 12:1–16
Bermejo C, Rodriguez E, Garcia R, Rodriguez-Pena J, Mlr DLC, Rivas C, Arias P, Nombela C, Posas F, Arroyo J (2008) The sequential activation of the yeast HOG and SLT2 pathways is required for cell survival to cell wall stress. Mol Biol Cell 19(3):1113
Bradford MM (1976) A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cadenas E, Davies KJ (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Bio Med 29(3):222–230
de la Cruz J, Llobell A (1999) Purification and properties of a basic endo-β-1,6-glucanase (BGN16.1) from the antagonistic fungus Trichoderma harzianum. Eur J Biochem 265(1):145–151
de la Cruz J, Pintor-Toro JA, Benitez T, LLobell A (1995) Purification and characterization of an endo-beta-1,6-glucanase from Trichoderma harzianum that is related to its mycoparasitism. J Bacteriol 177(7):1864–1871
Dowd JE, Riggs DS (1965) A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. J Biol Chem 240(2):863–869
Enisoglu-Atalay V, Atasever-Arslan B, Yaman B, Cebecioglu R, Kul A, Ozilhan S, Ozen F, Catal T (2018) Chemical and molecular characterization of metabolites from Flavobacterium sp. PLoS ONE 13(10):e0205817
Fayad K, Simao-Beaunoir AM, Gauthier A, Leclerc C, Mamady H, Beaulieu C, Brzezinski R (2001) Purification and properties of a β-1,6-glucanase from Streptomyces sp. EF-14, an actinomycete antagonistic to Phytophthora spp. Appl Microbiol Biotechnol 57(1–2):117–123
Fuchs BB, Mylonakis E (2009) Our paths might cross: the role of the fungal cell wall integrity pathway in stress response and cross talk with other stress response pathways. Eukaryot Cell 8(11):1616–1625
Ghasemi S, Safaie N, Shahbazi S, Shams-Bakhsh M, Askari H (2020) The role of cell wall degrading enzymes in antagonistic traits of Trichoderma virens against Rhizoctonia solani. Iran J Biotechnol 18(4):e2333
Gow NA, Latge J-P, Munro CA (2017) The fungal cell wall: structure, biosynthesis, and function. Microbiol Spectr 5(3):10–1128
Gusakov AV, Kondratyeva EG, Sinitsyn AP (2011) Comparison of two methods for assaying reducing sugars in the determination of carbohydrase activities. Int J Anal Chem 2011:283658
Gutierrez-Gongora D, Geddes-McAlister J (2021) From naturally-sourced protease inhibitors to new treatments for fungal infections. J Fungi 7(12):1016
Haas D, Defago G (2005) Biological control of soil-borne pathogens by fluorescent pseudomonads. Nat Rev Microbiol 3(4):307–319
Huang P, Cao H, Li Y, Zhu S, Wang J, Wang Q, Liu X, Lin F-C, Lu J (2022) Melanin promotes spore production in the rice blast fungus Magnaporthe oryzae. Front Microbiol 13:843838
Jin BJ, Chun HJ, Choi CW, Lee SH, Cho HM, Park MS, Baek D, Park SY, Lee YH, Kim MC (2024) Host-induced gene silencing is a promising biological tool to characterize the pathogenicity of Magnaporthe oryzae and control fungal disease in rice. Plant Cell Environ 47(1):319–336
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33(7):1870–1874
Li Z, Ye X, Liu M, Xia C, Zhang L, Luo X, Wang T, Chen Y, Zhao Y, Qiao Y (2019) A novel outer membrane β-1,6-glucanase is deployed in the predation of fungi by myxobacteria. ISME J 13(9):2223–2235
Liu X, Wang R, Bi J, Kang L, Zhou J, Duan B, Liu Z, Yuan S (2020) A novel endo-β-1,6-glucanase from the mushroom Coprinopsis cinerea and its application in studying of cross-linking of β-1,6-glucan and the wall extensibility in stipe cell walls. Int J Biol Macromol 160:612–622
Lu K, Chen R, Yang Y, Xu H, Jiang J, Li L (2023) Involvement of the cell wall–integrity pathway in signal recognition, cell-wall biosynthesis, and virulence in Magnaporthe oryzae. Mol Plant Microbe in 36(10):608–622
McElhaney RN (1975) Membrane lipid, not polarized water, is responsible for the semipermeable properties of living cells. Biophys J 15(8):777–784
Park H, Suh J, Lee S (2000) Ab initio studies on the catalytic mechanism of aspartic proteinases: nucleophilic versus general acid/general base mechanism. J Am Chem Soc 122(16):3901–3908
Plakys G, Urbelienė N, Urbelis G, Vaitekūnas J, Labanauskas L, Mažonienė E, Meškys R (2024) Conversion of β-1,6-glucans to gentiobiose using an endo-β-1,6-glucanase PsGly30A from Paenibacillus sp. GKG Chembiochem 25:e202400010
Pradeep N, Edison LK (2022) Microbial beta glucanases: molecular structure, functions and applications. Springer, Singapore
Qi Z, Liu M, Dong Y, Zhu Q, Li L, Li B, Yang J, Li Y, Ru Y, Zhang H, Zheng X, Wang P, Zhang Z (2016) The syntaxin protein (MoSyn8) mediates intracellular trafficking to regulate conidiogenesis and pathogenicity of rice blast fungus. New Phytol 209(4):1655–1667
Rast DM, Baumgartner D, Mayer C, Hollenstein G (2003) Cell wall-associated enzymes in fungi. Phytochemistry 64(2):339–366
Rezaie M, Aminzadeh S, Heidari F, Boojar MMA, Karkhane AA (2018) Biochemical characterization of recombinant thermostable Cohnella sp. A01 β-glucanase. Iran Biomed J 22(5):345
Ruiz-Herrera J, Ortiz-Castellanos L (2019) Cell wall glucans of fungi. A Review Cell Surf 5:100022
Shahriar SA, Imtiaz AA, Hossain MB, Husna A, Eaty M, Khatun N (2020) Rice blast disease. Annu Res Rev Biol 35(1):50–64
Takashima T, Komori N, Uechi K, Taira T (2023) Characterization of an antifungal β-1,3-glucanase from Ficus microcarpa latex and comparison of plant and bacterial β-1,3-glucanases for fungal cell wall β-glucan degradation. Planta 258(6):116
Tue NH, Phuc NH, Hoa PTB, Tien NQD, Loc NH (2024) Partitioning recombinant chitinase from Nicotiana benthamiana by an aqueous two-phase system based on polyethylene glycol and phosphate salts. Int J Biol Macromol 269:131924
Tupe S, Deshmukh SK, Zambare R, Tripathi A, Deshpande MV (2022) Biopolymers from fungi and their applications fungal biopolymers and biocomposites: prospects and avenues. Springer, pp 3–14
Wang D, Kim DH, Yun EJ, Park Y-C, Seo J-H, Kim KH (2017) The first bacterial β-1, 6-endoglucanase from Saccharophagus degradans 2–40 T for the hydrolysis of pustulan and laminarin. Appl Microbiol Biotechnol 101:197–204
Wang Y, Liu M, Wang X, Zhong L, Shi G, Xu Y, Li Y, Li R, Huang Y, Ye X (2021) A novel β-1,3-glucanase Gns6 from rice possesses antifungal activity against Magnaporthe oryzae. J Plant Physiol 265:153493
Yamamoto S, Kobayashi R, Nagasaki S (1974) Purification and properties of an endo β-1,6-glucanase from Rhizopus chinensis R-69. Agric Biol Chem 38(8):1493–1500
Ye X, Chen Y, Ma S, Yuan T, Wu Y, Li Y, Zhao Y, Chen S, Zhang Y, Li L (2020) Biocidal effects of volatile organic compounds produced by the myxobacterium Corrallococcus sp. EGB against Fungal Phytopathogens Food Microbiol 91:103502
Ye X, Li Z, Luo X, Wang W, Li Y, Li R, Zhang B, Qiao Y, Zhou J, Fan J (2020) A predatory myxobacterium controls cucumber Fusarium wilt by regulating the soil microbial community. Microbiome 8:1–17
Ye X, Liu W, Liao Y, Liu T, Zhao Y, Wang Y, Zhang Y, Li X, Xia C, Fang X (2022) Glycogen branching enzyme with a novel chain transfer mode derived from Corallococcus sp. strain EGB and its potential applications. J Agr Food Chem 70(15):4735–4748
Ye X, Xu C, Xie T, Zhang Y, Zhao Y, Xia C, Li Z, Huang Y, Fan J, Cao H (2023) Myxobacterial outer membrane β-1,6-glucanase induced the cell death of Fusarium oxysporum by destroying the cell wall integrity. Appl Environ Microbiol 89(1):e01236-e1322
Yu Q, Zhang B, Li J, Zhang B, Wang H, Li M (2016) Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radical Bio Med 99:572–583
Funding
This study was funded by the National Natural Science Foundation of China (Nos. 32272565, 32370119 and 32371730).
Author information
Authors and Affiliations
Contributions
T.X., and X.Y. were responsible for designing the methodology, investigating, writing the original draft and editing. J.S. and Z.G. were responsible for data analysis and validation. F.W. and Y.D. were responsible for formal confirmation. Y.L. and Z.C. were responsible for reviewing and editing.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, T., Shen, J., Geng, Z. et al. Antifungal characterizations of a novel endo-β-1,6-glucanase from Flavobacterium sp. NAU1659. Appl Microbiol Biotechnol 108, 437 (2024). https://doi.org/10.1007/s00253-024-13269-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13269-1