Abstract
Horses stay in different types of stables; especially during the cold season, they stay inside for most of the day. A stable is also a place where many people spend quite a lot of time either as employees who care for and train horses or as equine enthusiasts. Keeping horses in stables causes their constant exposure to high concentrations of particulate matter (PM) and molds in the air inside these facilities. The study was conducted in Udórz Stud Farm located in the southern region of Poland. It was carried out in two different types of stables: three runners and two box stables. The study continued for 2 years; samples were collected in each season of the year. The following devices were used: a six-stage Andersen-Graseby cascade impactor, the DustTrak™ II Aerosol Monitor 8530. The obtained results allowed for the conclusion that horses kept in box stables are exposed to lower concentrations of molds and yeasts than those kept in runners. Molds dominated in the stable air during humid periods—spring and autumn—while yeasts were more prominent during summer and winter. It was observed that cleaning stables reduces the morphotic elements of fungi in the air, even though it results in a higher level of particulate matter in the stable air. It should be noted that microclimate conditions were optimal for horses practically throughout the whole year.
Key points
• In stables, there is a high level of air intoxication, both by yeast and by mold fungi
• The concentrations of fungi in the air depend on the season and the stable cleaning procedure
• The PM concentrations depend on the type of stable
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, a significant increase in the incidence and severity of equine respiratory system disorders has been observed. This is the result of keeping horses in stables and riding them in closed rooms, which causes them to be constantly exposed to high concentrations of particulate matter (PM) in the air inside these facilities. Although horses are most often taken outside the stable for cleaning and feeding, the sedimentation rate of fine particles is so low that PM concentration is still high after horses return to the stable. PM in stables comes from bedding, feed, dried faeces, mites, and exfoliated animal cells; hence, up to 70% of PM is of organic origin (Mostafa et al. 2021). Particulate matter is a carrier for biological particles; hence, its concentration is correlated with the number of microorganisms creating bioaerosols (Nazarenko et al. 2018; Wolny-Koładka 2018; Lenart-Boroń et al. 2022). The level of particulate matter in the stable air is affected by many factors, such as the type of building, type and efficiency of ventilation, facility size, livestock density, type of feed, type of bedding, microclimatic conditions inside the stable, and climate where the stables are located (Witkowska et al. 2012; Siegers et al. 2018; Perrin 2021). Plant products used in stables—hay as feed and straw as bedding—contain a significant amount of autochthonous microflora, which can multiply in unfavourable storage conditions, so moldy feed and low-quality bedding become significant sources of mold spores. The size of the spores allows them to penetrate up to the level of the lung alveoli, where they may have infectious, toxic, and allergenic effects separately or in combination (Woolnough et al. 2015; Rick et al. 2016).
Due to their size, both dust particles and bioaerosol components can be divided into two fractions: total, with an aerodynamic diameter above 4.7 µm, which can settle in the upper respiratory tract, and respirable, which includes particles with a diameter below 4.7 µm. Particles with a diameter of 3.3–4.7 μm reach the trachea and primary bronchi, particles 1.1–3.3 μm reach the secondary bronchi and bronchioles, while particles smaller than 1.1 μm may reach the alveoli (Górny and Dutkiewicz 2002). The exact size of particles penetrating particular levels of the respiratory tract in horses is unknown. However, it is assumed that the abovementioned particles belonging to the respirable fraction reach the same place in the respiratory tree of humans, horses, and other animals (Hessel et al. 2009; Auger and Moore-Colyer 2017; Claußen and Hessel 2017).
This study aimed to determine how the concentrations of mold fungi and yeasts are influenced by different horse management systems, changing seasons and PM concentrations inside the stable. The obtained study results will be used to determine potential health threats to horses, grooms and equine enthusiasts.
Materials and methods
The study was carried out in Udórz Stud Farm. The facility is located in southern Poland. Four stables are located within the stud farm area. The first building includes two runner stables (R1 and R2), the second one another runner (R3), and two adjacent buildings include box stables (B1 and B2). The reference testing station (control) was located on the stud farm’s premises, approximately 50 m from the nearest stable. While the study was conducted, there were 84 horses in all stables. The characteristics of the stables and horses are presented in the article by Grzyb et al. (2022).
The measurements were taken over two calendar year periods—one measurement per each season of the year. The following months were selected as the most representative for a given season: April (spring), July (summer), September (autumn), and February (winter).
Bioaerosol samples were taken using a six-stage cascade impactor WES-710 model Andersen-Graseby (Westech Scientific Instrument, Hatfield Peverel, Essex, Great Britain). This impactor enables to divide bioaerosol stream into fractions based on the aerodynamic diameters of the particles: above 7 µm (F1); 4.7–7 µm (F2); 3.3–4.7 µm (F3); 2.1–3.3 µm (F4); 1.1–2.1 µm (F5), and 0.65–1.1 µm (F6). Fractions below 4.7 µm (F3-F6) are classified as respirable (RF).
The first measurement series were taken between 7:00 a.m. and 10:00 a.m.—before feeding and cleaning the stable (“Before bedding” (BB)), and the second series, after cleaning the stables, feeding horses, and adding fresh bedding (11:00 a.m.–2:00 p.m.) (“After bedding” (AB)).
Taking into account the location of the breathing zone of people and horses, air samples were collected 1.5 m above the ground (floor). The airflow rate through the impactor was constant and amounted to 28.3 dm3 per minute. The time for collecting air samples inside the stable ranged from 10 to 40 s; i.e., the volume of air passing through the impactor ranged from 4.7 to 18.9 dm3; at the control station (outside the stable), it took from 120 to 240 s (56.6–113.2 dm3).
MEA growth medium (Malt Extract Agar, Biomaxima, Lublin, Poland) was used for culturing fungi. The media were incubated for 5–7 days at 28 °C under aerobic conditions. After the incubation, colonies in Petri dishes were counted. Mold and yeast concentrations were calculated according to the following formula: L = [Pr·1000]/v, where L is the concentration of microorganisms in 1 m3 of air, Pr is the probable count of colonies according to the impactor manufacturer’s table, v is the volume of air taken by the impactor (dm3), 1000 is the converter to 1 m3. The results were presented as colony-forming units per 1 m3 of air (CFU/m3). The tests were taken in triplicate and the results were presented as medians of concentrations.
There are no guidelines regarding permissible concentrations of microorganisms in farm facilities, including stables. The obtained results were compared to the proposals of the Team of Experts in Biological Factors (ZECB) (Augustyńska and Pośniak 2016) on the recommended concentrations of microorganisms, treating stables as work premises contaminated with biological particulate matter (Table 1).
The DustTrak™ II Aerosol Monitor 8530 (TSI Inc., Shoreview, MN, USA) has interchangeable heads enabling the measurement of four fractions of particulate matter: PM10, PM4, PM2.5, and PM1 (i.e., PM particles with a diameter below 10, 4, 2.5, and 1 μm, respectively). The sampling time for each PM fraction was 180 s, and a single concentration reading was taken every 3 s, which gave a total of 60 independent measurements of average particulate matter concentrations.
Statistical analysis of the data was performed using the computer program Statistica data analysis software system, version 13.0 (TIBCO Software Inc., Santa Clara, CA, USA). After taking into account the fulfilment of the assumptions about the normality of the distribution of variables (Shapiro–Wilk’s test) and the homogeneity of variance (Levene’s test), the significance of differences between the means was verified with Kruskal–Wallis test. The values for which the probability of “p” was lower than 0.05 were considered statistically significant. The impact of particulate matter on the quantitative presence of fungi (molds and yeast) in the air was assessed using Spearman’s rank correlation coefficient, assuming statistically significant values at p < 0.05.
Results
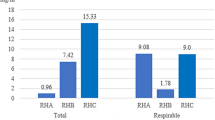
The obtained results were compared to the ZECB recommendations, and stables were treated as work premises contaminated with biological particulate matter. As presented in Table 2, 30% of air samples from the tested stables exceeded the recommended total fungal aerosol concentration (TC), while in the case of the respirable fraction (RF), as many as 55% of samples. The type of stable (runner vs. box) did not have a significant impact on the number of times the recommended concentrations were exceeded. Significantly more measurements with recommended concentrations exceeded were taken BB as compared to AB. Interestingly, a significantly higher number of exceedances was recorded before cleaning the stables: 55% for TC and as much as 85% for RF. After cleaning the stables, these indicators were 11 times lower for TC and 3.4 times for RF, respectively, compared to the measurements taken before cleaning.
It was observed that the median concentration of mold fungi in the stable air, regardless of the type of fungi, was more than twice as high before the stable cleaning as compared to the stable after cleaning (Table 3). In turn, the median fungal aerosol concentration for two types of stables, i.e., running stable (all R) and box stable (all B), before cleaning was almost identical and amounted to approximately 23,000 CFU/m3. When the stables were cleaned, a slightly higher median was observed in the case of box stables.
Table 4 presents data on yeast concentrations in the stable air. Based on median concentrations, it was found that, unlike molds, higher yeast concentrations occurred in box stables (all B), both before and after cleaning. Compared to the runners, the concentration differences were several per cent higher. As in the case of mold fungi, higher concentrations of yeast in the air occurred in stables BB. Compared to mold, the difference in the concentration of yeast in the air inside the stable buildings in comparison to the control station was even higher and ranged from 8 to 58 times.
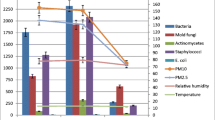
Using the Andersen-Graseby cascade impactor, the particle size distribution of fungal aerosol was also examined (Tables 5 and 6) in the tested farm facilities. It was found that the highest median mold concentrations were in the fine fraction F5 (2.2–1.1 µm) in both types of stables—before as well as after cleaning (Table 5). The lowest concentrations in the box stables (both BB and AB) and runners (AB) were of the ultrafine fraction penetrating the deepest into the respiratory system of both humans and horses (F6). In the runners before cleaning (BB), the thickest fraction F1 had the smallest share. Each of the fungal aerosol fractions in the air showed higher concentrations during measurements taken before cleaning the stables (BB); the difference ranged from 23 to 75% compared to AB measurements. The minimum and maximum mold concentrations were recorded at the control station (C) for fractions other than those inside the stable: fraction F4 had the highest concentrations and F2 the lowest. This was because different types of fungi, with different spore sizes, dominate in atmospheric air, and different ones in the air inside the stables. Yeast showed the highest concentrations and the largest share in the case of fraction F4 (Table 6): in the runners (before and after cleaning), in the box stable after cleaning and at the control station. Only in the case of the box stable before cleaning, the highest share of fraction F5 was recorded. The lowest proportion of yeast was found in fraction F6 in both types of stables, both AB and BB. At the control station, fraction F2 had the smallest share. It was observed that, as in the case of mold, higher yeast concentrations were also recorded during measurements taken before cleaning the stables; the differences ranged from 57 to 80% depending on a fraction.
In spring and autumn, there were more molds than yeasts in the air of the studied stables (Table 7) and at the control station (Table 8). The share of mold in this period ranged from 69.8 to 86.1%. In turn, in summer and winter, the air in the studied stables was dominated by yeast, whose share in these periods ranged from 60.3 to 71.4%.
An important factor indicating the occurrence of mycotoxin contamination is the index showing the degree of intoxication (inside/outside (I/O)) of the air in stables (Table 8); this phenomenon occurs when the concentration of microorganisms in the air inside the stable is higher than that in the atmospheric air outside the stable. High levels of intoxication caused by molds and yeasts were recorded especially in the winter before the stables cleaning (in the case of molds, they were up to 90 times higher compared to the control, and in the case of yeasts, 135 times higher). In winter, higher levels of intoxication were found in the runners. Slightly lower levels of intoxication (up to approximately 37 ×) in the case of molds and 59 × in the case of yeasts) were recorded in spring. The lowest level of intoxication occurred in the case of mold in the runners in autumn (on average about 9 ×).
When testing a particulate matter level in the stables, it was found that the highest concentrations were those of fraction PM10: in both types of stables, as well as at the control station (Table 9). A significant increase in the particulate matter level after cleaning the runners, ranging from 56.2% (fraction PM1) to 110.8% (PM10), is noteworthy. Dust particles in the stable air behave differently from fungal spores and yeast, the concentrations of which were lower after cleaning the stables; this proves their separate occurrence in the air. In the box stables, the increase of particulate air pollution was much lower than in the runners and ranged from 1.5 to 14.5% depending on a fraction. In the runners, after cleaning, the concentration of fraction PM1 increased the least, and PM10 the most; in the box stables, the trend was the opposite.
The impact of PM on the concentration of molds and yeast aerosols was assessed using Spearman’s rank correlation coefficient (Table 10). The analysis showed no significant correlation between the concentration of mold and the particulate matter of fractions: PM1.0, PM2.5, PM4.0, and PM10.0 (correlation coefficients respectively: R = 0.19, R = 0.20, R = 0.19, R = 0.18). The analysis also showed no significant correlation between the concentration of yeast and the particulate matter of fractions: PM1.0, PM2.5, PM4.0, and PM10.0 (correlation coefficients respectively: R = 0.14, R = 0.11, R = 0.14, R = 0.16).
Discussion
Air is a specific environment characterized by high variability of parameters, including those subject to mycological research. Measurement of the concentration of microorganisms taken in a specific environment characterizes its contamination at a specific moment (a specific point in time). Difficulties in interpretation are also caused by the lack of appropriate regulations regarding microbiological air quality. The absence of regulations is the result of, among other things, the lack of a simple relationship between the absorbed dose of bioaerosol and the health effect, but also the lack of a sufficient number of studies performed in various environments. The obtained research results are therefore related to the proposed norms drawn up by ZECB (Augustyńska and Pośniak 2016). There are not many studies on the mycological quality of air in stables, and an additional problem in comparing the obtained results is the use of different measuring devices.
Mold fungi and their morphotic elements can sensitize, can infect, and may have a toxic effect on both stable workers and horses (Górny and Dutkiewicz 2002). Dauvillier et al. (2018) proved that horses inhale fungal spores and mycelium fragments that are part of bioaerosol and that mold fungal elements have been found in horses respiratory tracts; this causes a significant increase in exposure to inflammatory airway disease (IAD) and RAO (recurrent equine obstructive disease). Fungi are not necessarily the primary cause of IAD, but when immune function is compromised (e.g., due to intense training or transport stress), they may affect horses’ ability to respond to infection. Similarly, in the case of healthy workers handling horses in stables, the morphotic elements of fungi may have a minor impact on their health, but factors such as a weakened immune system and treatment with corticosteroids or antibiotics may change it (Perrin 2021).
Mold concentrations in stables can vary significantly. This is influenced by many factors, such as location, type of stable, livestock density, type of ventilation, type of bedding, and frequency of stable cleaning (Budzińska et al. 2016). In our studies, mold concentrations in the box stables ranged from 1700 to 81,000 CFU/m3, while in the runners they ranged from 797 to 255,777 CFU/m3. The most similar values of fungal aerosol concentrations in box stables were found by Lenart-Boroń et al. (2022) (1370–112,100 CFU/m3) and Wålinder et al. (2011) (box stables: 200–96,000 CFU/m3). Budzińska et al. (2016) recorded several times lower fungi concentrations, ranging from 1270 CFU/m3 in winter to 47,800 CFU/m3 in autumn. A similar concentration range was shown by Dutkiewicz et al. (1994) (1700–28,000 CFU/m3). Even lower concentrations of airborne molds were observed by Witkowska et al. (2012) (1000–10,000 CFU/m3), and Wolny-Koładka (2018) (100 to 2400 CFU/m3).
Depending on the location of a given stable, different microclimatic conditions prevail inside each of them, which affects the fungi concentration. The distribution pattern of mold concentrations during different seasons may also vary (Auger and Moore-Colyer 2017; Nazarenko et al. 2018). Comparing our results with those obtained by Wolny-Koładka (2018), it was found that the highest mold concentrations in both cases were in summer (July), but the lowest were different: autumn (September) vs. winter (February). Both studies were performed in southern Poland.
When analyzing the distribution of mold spores in stable air, it was found that their largest share was recorded for the fine fraction F5 (2.2–1.1 µm), but the highest concentrations were in the range 4.7–1.1 µm (fractions F3–F5). The significant predominance of mold spores belonging to fraction F5 was confirmed by studies by Lenart-Boroń et al. (2022), as well as in these studies, fine and very fine fractions dominated (3.3–0.65 µm). Different results were presented by Mostafa et al. (2021), in whose study the highest concentrations of fungal spores were recorded for fractions ranging from > 11 to 2.1 µm, although they differed depending on the type of mold (Cladosporium > 11–2.1 µm, Wallemia 4, 7–2.1 µm, Eurotium > 11–4.7 µm, Aspergillus > 11–2.1 µm). It should be noted that these research were performed throughout the year (12 months) in the North Rhine-Westphalia (Germany).
Morphotic elements of fungi (mycelium fragments and spores) can float in the air on their own, but can also be attached to dust particles; therefore, when performing tests in a specific facility, it is important to simultaneously assess a particulate matter concentration. In our studies, we recorded PM10 concentrations in the box stables ranging from 49 to 740 µg/m3, while in the runners the range of particulate matter from this fraction was larger and ranged from 39 to 1110 µg/m3. Similar PM10 particulate matter concentrations were recorded in box stables by Wolny-Koładka (2018) 50–748 µg/m3. A much smaller range of concentrations, and at the same time several times lower maximum concentrations, were noted by Nazarenko et al. (2018) (62.8–125.7 µg/m3). In the case of fraction PM2.5, the concentration range in our studies was narrower than in Wolny-Koładka (2018) (38–387 µg/m3 vs. 48–654 µg/m3). The highest levels of particulate matter in both studies were found during the winter season. In autumn and winter, the type of fuel used to heat residential premises located in the vicinity of the stables, which are a subject of our studies, has a significant impact on the maximum concentrations of PM in the air. As in the case of fraction PM10, also for the finer fraction, PM2.5, Nazarenko et al. (2018) recorded the lowest particulate matter concentrations when straw was used as bedding (7–10 µg/m3), although it is believed that straw significantly affects the level of particulate matter, as proved by our research. Clements and Pirie (2007) found three times higher PM4 concentrations when straw was used as bedding compared to when bedding consisted of wood shavings.
In comparison to the results obtained by Lenart-Boroń et al. (2022), our results regarding particulate matter concentration in the box stables were at least two times higher; we recorded PM10 concentrations from 203 to 206 µg/m3 and for PM2.5 189–192 µg/m3. Values obtained by Lenart-Boroń et al. (2022) for PM10 were 10–109 µg/m3, and for fraction PM2.5, 9–86 µg/m3.
In our research, yeasts dominated the air in both types of stables (box stables and runners) in two seasons (summer and winter). The share of yeast in the total number of molds and yeasts ranged from 13.9 to 71.4%. No studies have been found in the available literature to compare yeasts and molds in stables. In studies carried out in hen houses, Sowiak et al. (2012) showed that yeast accounted for only 12% of all fungi, which was below the lower limit of the yeast share in our studies.
Polish legislation provides for the permissible concentration of PM10 and PM2.5 in the atmospheric air, but the limit value for the concentration during 1 day is only for PM10; it is 50 µg/m3. For fraction PM2.5, the pollution threshold refers to the calendar year. While taking most of the measurements, especially after cleaning the stables, the permissible concentration of PM10 was exceeded. According to Fiedorowicz (2007), the maximum particulate matter level in the stable cannot exceed 3 mg/m3; considering this value, the permissible PM10 concentration in the tested stables was not exceeded.
The conducted statistical analyses did not show a significant correlation between the concentrations of the tested microorganisms with the concentrations of any of the tested particulate matter fractions.
The conducted studies allowed for the conclusion that there is a high level of air intoxication in stables, both by yeast and by mold fungi. It has been proven that horses kept in box stables are exposed to lower concentrations of molds and yeasts (lower intoxication) compared to runners (Wolny-Koładka 2018). In spring and autumn, i.e., in the wetter periods, it was recorded that molds predominated in the stables, while in summer and winter yeasts prevailed. The fewest exceedances of the permissible concentration of fungi (in total: molds and yeasts) in the stable air occurred in autumn (TC and RF), while the highest levels for TC occurred in summer, and for RF in spring. It was observed that cleaning stables reduces the number of fungal morphotic elements in the air, even though during this process the level of particulate matter in stables increases. In the case of runners, the increase in PM is much higher than that in box stables. It should be emphasized that at such concentrations of fungi as determined during our studies carried out in the stables, fungal morphotic elements of fungi may pose a potential health threat to horses, staff, and people using horses.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Auger E-J, Moore-Colyer MJS (2017) The effect of management regime on airborne respirable dust concentrations in two different types of horse stable design. J Equine Vet Sci 51:105–109. https://doi.org/10.1016/j.jevs.2016.12.007
Augustyńska D, Pośniak M (eds) (2016) Harmful factors in the working environment – limit values. Interdepartmental Commission for Maximum Admissible Concentrations and Intensities for Agents Harmful to Health in the Working Environment, CIOP-PIB (in Polish)
Budzińska K, Szejniuk B, Jurek A, Michalska M, Traczykowski A, Berleć K (2016) Evaluation of selected physical and microbiological parameters of air in a boxstall stable. Acta Sci Pol Zootechnica 15(1):3–18. https://doi.org/10.21005/asp.2016.15.1.01
Claußen G, Hessel EF (2017) Particulate matter in equestrian stables and riding arenas. J Equine Vet Sci 55:60–70
Clements JM, Pirie RS (2007) Respirable dust concentrations in equine stables. Part 1: validation of equipment and effect of various management systems. Res Vet Sci 83:256–262. https://doi.org/10.1016/j.rvsc.2006.12.002
Dauvillier J, ter Woort F, van Erck-Westergren E (2018) Fungi in respiratory samples of horses with inflammatory airway disease. J Vet Intern Med 33:968–975. https://doi.org/10.1111/jvim.15397
Dutkiewicz J, Pomorski ZJH, Sitkowska J, Krysińska-Traczyk E, Skórska C, Prażmo Z, Cholewa G, Wójtowicz H (1994) Airborne microorganisms and endotoxin in animal houses. Grana 33(2):85–90
Fiedorowicz G (2007) Wymagania dotyczące warunków środowiskowych w chowie koni. Probl Agricult Eng 4:133–138 ((in Polish))
Górny RL, Dutkiewicz J (2002) Bacterial and fungal aerosols in indoor environment in Central and Eastern European countries. Ann Agric Environ Med: AAEM 9(1):17–23
Grzyb J, Podstawski Z, Bulski K (2022) Bacterial aerosol, particulate matter, and microclimatic parameters in the horse stables in Poland. Environ Sci Pollut Res 29:26992–27006. https://doi.org/10.1007/s11356-021-18142-6
Hessel EF, Garlipp F, Herman FA, van den Weghe I (2009) Generation of airborne particles from horse feeds depending on processing and type. J Equine Vet Sci 29:665–674. https://doi.org/10.1016/j.jevs.2009.07.013
Lenart-Boroń A, Bajor A, Tischner M, Kulik K, Kabacińska J (2022) Particulate matter concentrations and fungal aerosol in horse stables as potential causal agents in recurrent airway disease in horses and human asthma and allergies. Appl Sci 12(18):9375. https://doi.org/10.3390/app12189375
Mostafa E, Szabo E, Gates RS, Buescher W (2021) Identification of airborne particles and fungus spores concentrations within horses stables. Atmos Pollut Res 12(2):93–103. https://doi.org/10.1016/j.apr.2020.10.012
Nazarenko Y, Westendorf ML, Williams CA, Mainelis G (2018) The effects of bedding type in stalls and activity of horses on stall air quality. J Equine Vet Sci 67:91–98. https://doi.org/10.1016/j.jevs.2018.03.014
Perrin MH (2021) Effects of the air quality in equine stable environments on the respiratory health and allergy response of human personnel: a review. Honors theses and capstones. 582. https://scholars.unh.edu/honors/582
Rick EM, Woolnough K, Pashley CH, Wardlaw AJ (2016) Allergic fungal airway disease. J Investig Allergol Clin Immunol 26(6):344–354. https://doi.org/10.18176/jiaci.0122
Siegers EW, Anthonisse M, van Eerdenburg FJCM, van den Broek J, Wouters IM, Westermann CM (2018) Effect of ionization, bedding, and feeding on air quality in a horse stable. Vet Intern Med 32:1234–1240. https://doi.org/10.1111/jvim.15069
Sowiak M, Bródka K, Kozajda A, Buczyńska A, Szadkowska-Stańczyk I (2012) Fungal aerosol in the process of poultry breeding–quantitative and qualitative analysis. Med Pr 63:1–10. https://doi.org/10.1016/j.bbi.2015.03.008
Wålinder R, Riihimäki M, Bohlin S, Hogstedt C, Nordquist T, Raine A, Pringle J, Elfman L (2011) Installation of mechanical ventilation in a horse stable: effects on air quality and human and equine airways. Environ Health Prev Med 16:264–272. https://doi.org/10.1007/s12199-010-0195-5
Witkowska D, Kwiatkowska-Stenzel A, Jóźwiak A, Chorąży Ł, Wójcik A (2012) Microbiological contamination of air inside and around stables during different seasons of the year. Pol J Environ Stud 21(4):1061–1066
Wolny-Koładka K (2018) Microbiological quality of air in free-range and box-stall stable horse keeping systems. Environ Monit Assess 190:269. https://doi.org/10.1007/s10661-018-6644-0
Woolnough K, Fairs A, Pashley CH, Wardlaw AJ (2015) Allergic fungal airway disease: pathophysiologic and diagnostic considerations. Curr Opin Pulm Med 21(1):39–47. https://doi.org/10.1097/MCP.0000000000000129
Funding
This study was funded by the statutory activity 011100000-D111 of Department of Microbiology and Biomonitoring, University of Agriculture in Kraków, Poland.
Author information
Authors and Affiliations
Contributions
JG conceived, designed, and conducted the study. JG conducted the literature search and drafted the manuscript. ZP conceived, designed, and conducted the study. KB was involved in the analysis interpretation of data. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
N/A.
Consent for publication
N/A.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Grzyb, J., Podstawski, Z. & Bulski, K. Fungal aerosol and particulate matter in horse stables in Poland. Appl Microbiol Biotechnol 108, 426 (2024). https://doi.org/10.1007/s00253-024-13258-4
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13258-4