Abstract
The emergence and quick spread of the plasmid-mediated tigecycline resistance gene tet(X4) and colistin resistance gene mcr-1 have posed a great threat to public health and raised global concerns. It is imperative to develop rapid and accurate detection systems for the onsite surveillance of mcr-1 and tet(X4). In this study, we developed one-tube recombinase polymerase amplification (RPA) and CRISPR-Cas12b integrated mcr-1 and tet(X4) detection systems. We identified mcr-1- and tet(X4)-conserved and -specific protospacers through a comprehensive BLAST search based on the NCBI nt database and used them for assembling the detection systems. Our developed one-tube RPA-CRISPR-Cas12b-based detection systems enabled the specific detection of mcr-1 and tet(X4) with a sensitivity of 6.25 and 9 copies within a detection time of ~ 55 and ~ 40 min, respectively. The detection results using pork and associated environmental samples collected from retail markets demonstrated that our developed mcr-1 and tet(X4) detection systems could successfully monitor mcr-1 and tet(X4), respectively. Notably, mcr-1- and tet(X4)-positive strains were isolated from the positive samples, as revealed using the developed detection systems. Whole-genome sequencing of representative strains identified an mcr-1-carrying IncI2 plasmid and a tet(X4)-carrying IncFII plasmid, which are known as important vectors for mcr-1 and tet(X4) transmission, respectively. Taken together, our developed one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems show promising potential for the onsite detection of mcr-1 and tet(X4).
Key points
• One-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems were developed based on identified novel protospacers.
• Both detection systems exhibited high sensitivity and specification with a sample-to-answer time of less than 1 h.
• The detection systems show promising potential for onsite detection of mcr-1 and tet(X4).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Antibiotics have been widely used in animal husbandry for recent several decades as an important means to treat and prevent microbial infections and promote animal growth (Woolhouse and Ward 2013; Zhu et al. 2017). However, the long-term overuse of antibiotics has been causing the emergence and spread of antibiotic-resistant pathogens, particularly multidrug-resistant ones, in livestock and poultry farms and their meat products (Serwecińska 2020; Teillant and Laxminarayan 2015; VT Nair et al. 2018). Such contaminated meat products could lead to infections in humans and cause diseases, such as diarrhea, which can seriously threaten public health (Exner et al. 2017; Manyi-Loh et al. 2018). The increasing spread of antibiotic-resistant pathogens has posed great challenges to the treatment of microbial diseases using antibiotics.
In the absence of new suitable antibiotics, colistin and tigecycline are currently the “last line of defense” antibiotics for the clinical treatment of multidrug-resistant Gram-negative bacteria (Lu et al. 2021; Osei et al. 2016). However, in recent years, their antibiotic-resistance genes (ARGs) have also been continuously discovered and reported. In 2015, Liu et al. first discovered a plasmid-mediated colistin resistance gene from animal-derived Escherichia coli and named it mcr-1 (Liu et al. 2016). They simultaneously proved that mcr-1 could be transferred to Klebsiella pneumoniae through the IncI2 conjugative plasmid and mediate colistin resistance in the receipt strain. Since then, at least 10 homologues of mcr have been described; however, mcr-1 is the most prevalent mcr variant identified in clinics worldwide (Dadashi et al. 2022). Among the identified tet(X) variants that can degrade tigecycline, tet(X4) is recognized as a highly prevalent plasmid-borne variant that exhibits high enzymatic activity and can mediate high resistance to tigecycline (16–32 mg/mL) (He et al. 2019). To date, tet(X4)-positive strains have been identified in animals (Wang et al. 2022a, b), foods (Sun et al. 2021), the environment (Cui et al. 2020), and humans (Cui et al. 2022; Zhang et al. 2022a, b). Gradually, these strains are increasingly being reported worldwide.
The rapid spread of mcr-1 and tet(X4), which is exacerbated by the self-replication and transfer of bacterial interstitial plasmids, has raised serious concerns regarding the safety of livestock and poultry industries and human health (He et al. 2019; Liu et al. 2016). The food chain, one of the main transmission routes of ARGs, is responsible for the transmission of mcr-1 and tet(X4) to humans through livestock and poultry meat products. This may have adverse effects on human health, such as the imbalance of gut microbiota and the proliferation of antibiotic-resistant pathogens (de Mesquita et al. 2022). A study on the prevalence of mcr-1 in Enterobacteriaceae species isolated from the United Kingdom (UK) identified four mcr-1-positive human-derived Salmonella strains that shared the same ST type as swine-derived Salmonella strains reported in a UK farm, indicating the potential transmission of these four strains through the food chain, particularly through animal food (Doumith et al. 2016). Similarly, tet(X4) has been isolated from various animals, such as pigs, chickens, and ducks, in more than 23 countries, with pigs being the primary source of animal isolation (Zhang et al. 2022a, b). For instance, Sun et al. identified 25 tet(X4)-positive strains from a total of 311 retail meat samples, with pork accounting for 52% of the isolates (Sun et al. 2021). Moreover, in a study conducted in Singapore, 10.1% of 109 residents carried tet(X4)-positive strains in their feces, indicating that tet(X4) has been widely distributed in the gut microbiota of healthy residents in Singapore (Zhang et al. 2022a, b). Alarmingly, an increasing number of studies have revealed the coexistence of tet(X4) and mcr-1 in E. coli (Li et al. 2022a, b, c, d, e). The convergence of resistance to “last line of defense” antibiotics can lead to the potential emergence of superbugs in the future. Therefore, the rapid and accurate onsite detection of mcr-1 and tet(X4) in food chain-associated samples, such as meat and associated environments, is crucial for ensuring food safety and maintaining human health.
In recent years, various methods have been developed for the detection of mcr-1 and tet(X4); these include cultivation-based assays, PCR-based detection techniques (e.g., PCR-dipstick chromatography and real-time quantitative PCR assays) (Coppi et al. 2018; Shanmugakani et al. 2019; Chabou et al. 2016; Li et al. 2020), and other modern molecular diagnostic techniques. Nonetheless, despite its advantages in visualization and reliability, the cultivation-based assays are characterized by low sensitivity and time-consuming procedures. Concurrently, PCR-based techniques, while possessing high specificity, may presently be unsuitable for onsite detection due to their reliance on specialized amplification instruments (e.g., a thermocycler) and trained laboratory professionals. Recently, the CRISPR-Cas-based nucleic acid detection technology has shown promising potential for rapid onsite pathogen and ARG detection. This technology offers several advantages, including ease of operation, independence from specialized amplification instruments, and superior accuracy and specificity compared to other molecular detection technologies (Li et al. 2023; Zhao et al. 2023; Wang et al. 2022a, b). Cas proteins, such as Cas12a and Cas12b, exhibit collateral cleavage activity that can activate the cleavage domain upon target nucleic acid cleavage, leading to the unrestricted cleavage of free nucleic acids present in the system (Li et al. 2019). Based on this principle, the results can be determined by the presence or absence of fluorescence by adding free fluorescent probes to the system. Cas12b, having a molecular weight inferior to that of Cas9 and Cas12a, is one of the most widely used Cas proteins for developing CRISPR-Cas-based nucleic acid detection systems. Cas12b exhibits a preference for recognizing protospacer sequences with the PAM site “TTA” (Yang et al. 2016). Moreover, its diminutive size coupled with a low tolerance for even a single-base mismatch renders it a favorable candidate for nucleic acid detection in vitro (Varshney et al. 2021). Currently, Cas12b has been used to rapidly and accurately detect pathogenic bacteria, such as Campylobacter jejuni (sample-to-answer time < 40 min) (Huang et al. 2021), Mycobacterium tuberculosis complex (< 70 min) (Yang et al. 2023), Pseudomonas aeruginosa (< 1 h) (Qiu et al. 2023), and Klebsiella pneumoniae (Qiu et al. 2022). Moreover, nucleic acid amplification technologies, such as recombinase polymerase amplification (RPA), which is a rapid amplification technology that can exponentially amplify the target sequence within 30 min, can be applied to the CRISPR-Cas-based detection systems to further enhance the specificity and sensitivity of detection (Hijjawi et al. 2023). The combination of RPA and CRISPR-Cas12a can enable the rapid detection of methicillin-resistant Staphylococcus aureus, achieving the detection of as low as 10 copies of the target gene within only 20 min (Li et al. 2022a, b, c, d, e). In this study, we developed an RPA-CRISPR-Cas12b-based detection technique to offer a novel approach for the rapid and accurate onsite identification of ARGs, such as mcr-1 and tet(X4).
The combination of a CRISPR-Cas-based detection system and RPA is typically achieved by adding reagents in a stepwise manner and waiting for RPA to react before introducing CRISPR components (Lin et al. 2022). However, this approach may result in aerosol contamination and generate false-positive results (Lin et al. 2022). Moreover, the stepwise approach lacks convenience for onsite detection purposes. In contrast, colocalizing RPA- and CRISPR-Cas-based detection assays within the same reaction vessel can mitigate the risk of cross-contamination resulting from the opening and closing of PCR tubes during the experimental process (Hu et al. 2022). In this study, specific and conserved protospacer sequences used for the CRISPR-Cas12b assay were identified in mcr-1 and tet(X4) by a comprehensive BLAST search based on the NCBI nt database, and the primer pairs for the RPA assay were then designed. Finally, one-tube RPA- and CRISPR-Cas12b-colocalized mcr-1 and tet(X4) detection systems were developed. The feasibility of the two developed systems was assessed based on extracted DNA samples, artificially contaminated pork samples, and pork and their associated environmental samples collected from retail markets. The one-tube RPA-CRISPR-Cas-based mcr-1 and tet(X4) detection systems accepted crude DNA prepared by boiling, thereby eliminating the need for bacterial isolation and DNA purification. The turnaround time from sample to result was ~ 55 min and ~ 40 min for mcr-1 and tet(X4), respectively. The developed one-tube RPA-CRISPR-Cas-based detection systems showed promising potential for the onsite detection of mcr-1 and tet(X4).
Materials and methods
Strains and oligonucleotides
The tet(X4)-positive strain E. coli 3934, the mcr-1-positive strain E. coli MCE9, and several other tet(X4)- and mcr-1-negative strains, including E. coli ATCC25922, C. jejuni NCTC11168, S. aureus ATCC27217, Salmonella Enteritidis C50041, S. Typhimurium SL1344, and Listeria monocytogenes EGD-e, were used for assessing the CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems.
DNA oligonucleotides and ssDNA-FQ fluorescent probes were synthesized by GenScript Biotechnology (Nanjing, China) (Table 1).
Design and preparation of sgRNAs
In total, 36 currently known mcr-1 variants were downloaded (Table S1) (Che et al. 2023). The 383 tet(X4) sequences available in the NCBI MicroBIGG-E database (as of October 20, 2022) were downloaded, and 9 sequence variants were identified using cd-hit-est with parameter -c 1 (Table S2). The mcr-1 and tet(X4) sequences were aligned using MEGA X software to identify their conserved regions. The completely conserved sequence regions with a Cas12b-preferring “TTA” PAM motif were then identified in the aligned mcr-1 and tet(X4) sequences, and the 20 bp protospacer sequences were extracted accordingly. Following this, the protospacer sequences were blasted against the nt database (91,700,559 sequences; total length, 1.075 Tb; database construction date, March 27, 2023) using BLASTN with the parameters “-task blastn-short, 100% identity, and 100% coverage.” The sequences containing the protospacers were then extracted from the nt database and compared with mcr-1 or tet(X4) sequences to determine the specificity and conservation of the protospacers. Finally, a protospacer sequence that exhibited high conservation and specificity (1104 hits in the nt database, of which 1102 sequences were mcr-1 positive and two were from irrelevant eukaryotic genomes, namely Campaea margaritaria and Yponomeuta rorrellus) was identified for mcr-1 (Fig. S1A and Table 1). Similarly, a highly conserved and specific protospacer was identified for tet(X4) (205 hits in the nt database, of which 202 sequences were tet(X4) positive, one was only 93 bp long and was therefore excluded from further analysis, and two were from irrelevant eukaryotic genomes, namely Apotomis turbidana and A. betuletana) (Fig. S1B and Table 1).
The two specific protospacers for mcr-1 and tet(X4) were cloned into the pUC18 vector and amplified using the mcr-sg-F and mcr-sg-R primers and tet(X4)-sg-F and tet(X4)-sg-R primers, respectively. PCR products were purified using the TaKaRa MiniBEST Agarose Gel DNA Extraction Kit (TaKaRa, Japan) and used as the template for sgRNA biosynthesis using the Transcriptaid T7 Transcription Kit (Thermo Fisher Scientific, USA) by incubating at 37 °C for 12 h. To digest the remaining DNA fragments, DNase I was added to the aforementioned reaction solution and incubated at 37°C for 30 min. Then, the transcribed sgRNA-mcr-1 and sgRNA-tet(X4) of mcr-1 and tet(X4), respectively, were purified using the RNA Clean & Concentrator-5 Kit (Zymo Research, USA) and stored at − 80 °C.
Assessment of target cleavage activity of Cas12b protein and assembly of CRISPR-Cas12b-based detection systems
The Cas12b (AacCas12b) gene was amplified from Alicyclobacillus acidoterrestris strain, cloned into the pET-28a vector, then transformed into E. coli strain BL21 (DE3). The subsequent Cas12b expression and purification processes were conducted following the methodology outlined in our prior study (Huang et al. 2021). To verify the cis-cleavage activity of the Cas12b protein, we amplified a 1000 bp region containing the specific protospacer for mcr-1 and tet(X4) from E. coli MCE9 and E. coli 3934 using primer set mcr-1JF/ mcr-1JR and tet(X4)-1JF/ tet(X4)-1JR, respectively. The specific protospacer sequences were located at ~ 750 bp within the PCR products. The PCR assays commenced with an initial denaturation step at 98 °C for 3 min, followed by 30 cycles of denaturation at 98 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min, and a final extension step at 72 °C for 10 min. The mcr-1 and tet(X4) PCR product was mixed with Cas12b and the obtained transcribed sgRNA-mcr-1 and sgRNA-tet(X4), respectively, and the mixture was incubated at 48 °C for 30 min. Following this, 3 mL of 6 × Cas-STOP Loading Buffer was added to the mixture and incubated at 65 °C for 5 min before separation by gel electrophoresis.
To verify the trans-cleavage activity of the Cas12b protein, the CRISPR-Cas12b-based detection systems targeting mcr-1 and tet(X4) were prepared in three steps. In step A, a 20 µL reaction system containing 2 µL Reaction NEBuffer 2.1 (10 ×), 0.5 µL Cas12b (250 nM), 0.5 µL sgRNA-mcr-1/sgRNA-tet(X4) (250 nM), 2 µL target DNA (should contain more than 10 copies of targets, 6.25 × 108 copies for mcr-1 and 9 × 108 copies for tet(X4) in this study), 1 µL ssDNA-FQ probe sensor 5’- 6FAM-N12-3’-BHQ1 (500 nM), and RNase-free water was set up. In step B, the reaction system was mixed and incubated at 48 °C for 30 min and then incubated at 65 °C for 5 min. In step C, when the target DNA matched the specific protospacers of mcr-1 and tet(X4), the fluorescence intensity was assessed and captured using a Dual LED Blue/White Light Transilluminator (Solarbio, China) under 485 nm light. The resulting images were saved and analyzed using ImageJ to calculate fluorescence values.
Assessment of specificity of CRISPR-Cas12b-based detection systems
To assess the specificity of the CRISPR-Cas12b-based systems for detecting mcr-1 and tet(X4), the DNA samples of E. coli MCE9, E. coli 3934, Salmonella, L. monocytogenes, and several other foodborne pathogenic bacteria were used as target templates. The DNA samples were added to the CRISPR-Cas12b-based detection systems, and the presence of a green fluorescence signal for each reaction system was assessed under 485 nm light.
RPA primer design and reaction conditions
RPA primer pairs were designed to amplify the protospacer-containing regions of mcr-1 and tet(X4). Three sets of RPA primer pairs were obtained for mcr-1 and tet(X4), respectively (Table 1). To evaluate the efficacy of the designed primer pair sets, an RPA reaction was performed using the DNA Isothermal Temperature Rapid Amplification Kit (Amplification Future, China). In total, 50 µL of the reaction mixtures included 29.4 µL of buffer A solution, 2 µL of a forward primer, 2 µL of a reverse primer, 12.1 µL of nuclease-free ddH2O, 1 µL of genomic DNA (50 ng/µL), and 2.5 µL of buffer B added into 0.2 mL PCR tubes. The mixtures were subsequently incubated at 39°C for 30 min, followed by purification with an equal volume of phenol/chloroform and centrifugation at 12,000 rpm for 5 min. From the resulting eluate, 3 µL of the PCR product was subjected to 1% agarose gel electrophoresis for 30 min to assess its quality and purity.
Assembly of one-tube RPA-CRISPR-Cas12b-based detection systems
While the optimal reaction temperature for RPA is 37–42 °C, Cas12b exhibits enzymatic activity within a relatively wide temperature range; therefore, we determined the reaction temperature for the one-tube RPA-CRISPR-Cas12b-based detection systems. First, a two-step approach was employed to screen the optimal amplification primers for the RPA assay (Aman et al. 2022). Specifically, for each gene, the PCR products amplified using the three sets of RPA primers were introduced into the CRISPR-Cas12b-based detection systems. The primer pair set that yielded the best performance in the aforementioned step was selected for the one-tube RPA-CRISPR-Cas12b-based detection systems. Second, the optimal reaction temperature for the one-tube assay was determined. The reaction system for the one-tube assay consisted of 14.7 µL of buffer A, 1.25 µL of buffer B, 1 µL of a forward primer, 1 µL of a reverse primer, 2 µL of genomic DNA (50 ng/µL), 2 µL of Reaction NEBuffer 2.1 (10 ×), 0.5 µL of Cas12b, 0.5 µL of sgRNA-mcr-1/sgRNA-tet(X4), 1 µL of ssDNA-FQ probe sensor, and RNase-free water. Gradient temperatures of 37 °C, 38.5 °C, 42 °C, 43.3 °C, and 45 °C were established to evaluate the performance by determining the fluorescence density values after a 1 h reaction period. The optimal primers identified using the two-step approach may not necessarily exhibit the best amplification efficiency in the one-tube detection systems (Aman et al. 2022). Hence, the one-tube detection systems were finally configured using the three primer pairs targeting mcr-1 and tet(X4) at the determined optimal reaction temperatures. Their performance was evaluated based on the fluorescence values obtained at 15-min intervals over a 1-h reaction period.
Assessment of the sensitivity of one-tube RPA-CRISPR-Cas12b-based detection systems
To assess the sensitivity of the one-tube RPA-CRISPR-Cas12b-based detection systems, the mcr-1- or tet(X4)-specific protospacer-containing pUC18 vector was diluted by 1:10 serial dilutions. Subsequently, 2 µL of the resulting solution was utilized as the DNA template in the one-tube detection systems. Following the completion of the reaction, green fluorescence was visually assessed under 485 nm light. The fluorescence density values were analyzed using ImageJ.
Assessment of one-tube RPA-CRISPR-Cas12b-based detection systems by using pork samples spiked with mcr-1 and tet(X4) contamination.
Fresh pork samples were procured from a local retail market in Yangzhou and subsequently diced into approximately 2.5 g portions. The diced samples were then treated with ultraviolet light for 45 min within a biosafety cabinet with periodic flipping every 5 min. The samples were then washed twice with ddH2O to eliminate any potential interference from pork-carried mcr-1 and tet(X4) during the detection process. Following this, 2.5 mL of E. coli DH5M containing the mcr-1-carrying plasmid pUC18 or E. coli DH5T containing the tet(X4)-carrying plasmid pUC18 (OD600 = 1) was inoculated into 22.5 mL of LB broth to generate the initial inoculants (OD600 = 0.1). The inoculants were sequentially diluted by 1:10 serial dilutions. Subsequently, 2.5 g of cleaned pork samples were placed into sampling bags containing varying dilutions of bacterial solutions and incubated at 37 °C under 120 rpm for 1 h. The pork samples were then removed from the bacterial solutions, dried using sterilized filter paper, and transferred to new sampling bags containing 22.5 mL PBS solution. After homogenization for 2 min, 10 µL of bacterial solution was collected for bacterial cultivation and 1 mL of bacterial solution was used for DNA extraction. The solution was centrifuged at 8000 rpm for 5 min, and the bacterial pellet was then washed twice with PBS and resuspended in 50 µL nuclease-free water. The bacterial suspension was boiled at 100 °C for 10 min and centrifuged at 8,000 rpm for 5 min. The resulting supernatant was aspirated as a DNA template for the detection systems. The populations of mcr-1-positive E. coli DH5M and tet(X4)-positive E. coli DH5T in the contaminated pork samples were also determined using the gradient dilution plating method.
Evaluation of one-tube RPA-CRISPR-Cas12b-based detection systems based on pork and environmental samples collected from retail markets
To evaluate the practical efficacy of our newly developed one-tube RPA-CRISPR-Cas12b-based systems for detecting mcr-1 and tet(X4), a total of 40 pork samples and environmental samples (including chopping boards, workbenches, and 100-cm2 areas on the ground in the raw meat sales area) were collected from three retail markets in Yangzhou, Jiangsu, China, in May 2022. After each sample was mixed well with PBS, 1 mL of the sample was transferred into a 1.5 mL centrifuge tube and centrifuged at 8000 g for 5 min. The PBS washing step was repeated twice, followed by resuspension in 50 µL of nuclease-free water. The resulting suspension was then boiled at 100 °C for 10 min and centrifuged at 5,000 rpm for 5 min. The obtained supernatant served as the input template DNA for the one-tube RPA-CRISPR-Cas12b-based detection systems to determine whether the samples were contaminated with mcr-1 or tet(X4).
Furthermore, bacterial cultivation were performed to reveal the mcr-1 and tet(X4) contamination rates in the pork and environmental samples. The mcr-1 and tet(X4)-specific primer sets described by Liu et al. (2016) and He et al. (2019) were used for the mcr-1 and tet(X4) detection PCR assays, respectively. The DNA templates obtained from the belowmentioned colonies were used as input templates in the assays. The PCR products were verified by Sanger sequencing. Colistin- and tigecycline-resistant strains were isolated using an optimized method described by Li et al. (2022a, b, c, d, e), respectively. In brief, the collected samples were placed in centrifuge tubes containing 5 mL of peptone-buffered water and incubated at 37 °C for 24 h. Then, the bacterial suspensions were sequentially diluted by 1:10 serial dilutions. Subsequently, 100 µL of the 10 − 4 gradient was absorbed and spread on MH2 agar containing 2 mg/L colistin and on that containing 2 mg/L tigecycline. The plates were then incubated at 37°C for 12–16 h. Typical single colonies with inconsistent morphology were selected by colony PCR to determine the presence of mcr-1 or tet(X4). The taxonomy of the identified mcr-1-positive and tet(X4)-positive strains was determined based on their 16S rDNA sequences.
Whole-genome sequencing and bioinformatics analysis of mcr-1- and tet(X4)- positive strains.
From the obtained mcr-1- and tet(X4)-positive strains, two representative strains (one mcr-1-positive strain and one tet(X4)-positive strain) were selected for whole-genome sequencing using an Illumina and Nanopore hybrid sequencing approach. In brief, genomic DNA was extracted using the QIAamp® PowerSoil® Pro Kit (Qiagen, Germany), according to the manufacturer’s protocol. The DNA concentration and integrity were assessed using 0.8% agarose gel electrophoresis, and Qubit 4.0 (Thermo Fisher Scientific, USA). High-quality long reads generated by Nanopore sequencing (Q > 7 and length > 1 kb) were assembled using Flye (v2.8.3) (Kolmogorov et al. 2019), and the assembled contigs were polished using NextPolish (v2) (Hu et al. 2020) software based on the Illumina short reads. The plasmid replicons were identified using PlasmidFinder implemented in the CGE platform based on the Enterobacteriaceae database with the following parameters: minimum 95% identity and 85% query coverage (Carattoli et al. 2014). The plasmid sequences containing mcr-1 and tet(X4) were blasted against the NCBI nt database, and the closely-related plasmid sequences in the nt database were extracted and compared using BRIG (Alikhan et al. 2011). ARGs were also predicted from the mcr-1- and tet(X4)-positive plasmids using Resistance Gene Identifier (RGI, v5.1.0) based on the Comprehensive Antibiotic Resistance Database (CARD) (Alcock et al. 2020). The genomic sequences of the two isolates have been deposited in the NCBI database under the accession numbers CP126999-CP127002 for strain YZ2-2 and CP127003-CP127008 for strain YZ3-11, respectively.
Results
Assembly and specificity assessment of CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems
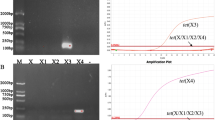
The mcr-1- and tet(X4)-conserved and -specific protospacer sequences used for assembling the CRISPR-Cas12b-based detection systems were identified using a comprehensive BLAST approach. In brief, after the representative mcr-1 and tet(X4) sequences were aligned, the Cas12b-preferred PAM motif “TTA” and the corresponding protospacers were identified in the conserved regions of the aligned mcr-1 and tet(X4) sequences. The conversation and specificity of the identified protospacers were then determined by blasting against the NCBI nt database (see “Materials and methods”). Finally, a protospacer that was conserved in all 1042 identified mcr-1 sequences deposited in the NCBI nt database but was only identified in two irrelevant eukaryotic genomes was used for the assembly of the CRISPR-Cas12b-based mcr-1 detection system (Fig. S1A). Similarly, a tet(X4)-conserved and -specific protospacer that was conserved in 202 tet(X4) sequences and three non-tet(X4)-originating sequences identified based on the nt database was also identified. The sgRNAs were synthesized based on the two identified protospacer sequences, and the CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems were consequently assembled. Then, the sensitivity and specificity of the assembled detection systems were evaluated. Specifically, 1000 bp sequence amplicons of mcr-1 and tet(X4) were amplified, with protospacer targets being located at 750 bp in the PCR product. Subsequently, each PCR product was incorporated into the detection systems to assess the cleavage activity of the Cas12b protein. The mcr-1 and tet(X4) PCR products were successfully cleaved into two fragments with expected sizes of approximately 250 and 750 bp, respectively, indicating that sgRNAs of mcr-1 and tet(X4) could effectively guide Cas12b to cis-cleave the target sequence (Fig. 1 A and B). Upon the addition of the ssDNA-FQ fluorescent probe, a robust green fluorescence signal was observed for both detection systems (Fig. 1 C and D). Collectively, these results demonstrate the feasibility of utilizing the CRISPR-Cas12b-based systems for detecting mcr-1 and tet(X4).
We then selected DNA samples from the tet(X4)-positive strain E. coli 3934, the mcr-1-positive strain E. coli MCE9, and several other zoonotic bacterial pathogens that do not contain mcr-1 and tet(X4), including E. coli ATCC25922, C. jejuni NCTC11168, S. Enteritidis C50041, S. Typhimurium SL1344, S. aureus ATCC27217, and L. monocytogenes EGD-e, to evaluate the specificity of the CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems. The results demonstrated that only the tube containing the DNA of the mcr-1-positive strain E. coli MCE9 exhibited a strong green fluorescence signal for the mcr-1 detection system (P < 0.05, ANOVA) (Fig. 2 A and B); however, no discernible green fluorescence was noted in other tubes containing DNA samples from non-mcr-1 strains. A similar result was observed for the tet(X4) detection system; only the tet(X4)-positive strain E. coli 3934 could activate the system (P < 0.05, ANOVA) (Fig. 2 C and D). These results clearly demonstrate that the CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems could specifically recognize mcr-1- and tet(X4)-derived DNA in the DNA samples.
The specificity assessment and fluorescence intensity of the CRISPR-Cas12b detection system for mcr-1 (A and B) and tet(X4) (C and D) detection, respectively. Different letters on the top of columns denote significant differences (P < 0.05, ANOVA). Error bars represent means ± SEM (n = 3 replicates). In A and B, sample 1 represents the mcr-1-positive strain E. coli MCE9; samples 2–7 represent several zoonotic bacterial pathogens that do not contain mcr-1, including E. coli ATCC25922, C. jejuni NCTC11168, S. Enteritidis C50041, S. Typhimurium SL1344, S. aureus ATCC27217, and L. monocytogenes EGD-e; and sample 8 represents negative control; in C and D, sample 1 represents the tet(X4)-positive strain E. coli MCE9; samples 2–7 represent several zoonotic bacterial pathogens that do not contain tet(X4), including E. coli ATCC25922, C. jejuni NCTC11168, S. Enteritidis C50041, S. Typhimurium SL1344, S. aureus ATCC27217, and L. monocytogenes EGD-e; and sample 8 represents negative control. The “background subtracted fluorescence” values were fluorescence values recorded in the reaction tubes minus the average fluorescence value obtained from three blank tubes
Development of one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems
Previous studies have demonstrated that the combination of RPA and CRISPR-Cas-based detection systems can improve detection sensitivity and specificity (Hijjawi et al. 2023). We then tried to employ RPA in the CRISPR-Cas12b-based detection systems to enhance the efficacy of the systems for mcr-1 and tet(X4) detection. The RPA primer pairs were designed based on the aligned mcr-1 and tet(X4) sequences, with the protospacer sequence being located in the central part of the amplified regions. Three pairs were designed for mcr-1 and tet(X4) each. All the designed primer pairs could amplify the target regions; however, the amplification efficiency varied among the primer pairs (Fig. S2). For instance, the mcr-1 primer pair set 2 exhibited lower amplification efficiency than the primer pair sets 1 and 3 (Fig. S2A), while the tet(X4) primer pair set 3 produced a relatively higher concentration of PCR products than the other two primer pair sets (Fig. S2B). Because of the inconsistent amplification efficiency of each RPA primer pair, a two-step approach was employed to screen the optimal primers for mcr-1 and tet(X4). For mcr-1, the primer pair set mcr-RPA-2 that exhibited low amplification efficiency was eliminated from further analysis (Fig. S2A). Subsequently, RPA was performed on the primer pair sets mcr-RPA-1 and mcr-RPA-3. The amplified products were then added to the CRISPR-Cas12b-based detection system. The product from the primer pair set mcr-RPA-3 could more efficiently activate the detection system and generate a more intense fluorescent signal than that from the primer pair set mcr-RPA-1 (P < 0.05, ANOVA) (Fig. S3A). Therefore, mcr-RPA-3 was identified as the optimal primer pair set for the RPA-CRISPR-Cas12b-based mcr-1 detection system. The tet(X4) detection system containing the amplification product of the three tet(X4) primer pairs exhibited strong fluorescence signals. In particular, the fluorescence signal from the primer pair set tet-RPA-1 was slightly but significantly higher than that from the other two primer pair sets (P < 0.05, ANOVA) (Fig. S3B). Thus, tet-RPA-1 was identified as the optimal primer pair set for detecting tet(X4) by combining RPA and CRISPR-Cas12b.
As the optimal temperature of RPA and Cas12b is different (RPA operates optimally at 37–42 °C and Cas12b at 45–55 °C), the optimal reaction temperature for the one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems was determined at five representative temperatures ranging from 37 to 45 °C (37 °C, 38.5 °C, 42 °C, 43.3 °C, and 45 °C). For the mcr-1 detection system, the fluorescence density gradually increased when the temperature increased from 37 to 43.3 °C. The fluorescence density slightly decreased at 45 °C compared with that at 43.3 °C (P < 0.05, ANOVA) (Fig. 3A). These results suggest that the activity of Cas12b gradually augmented with an increase in temperature; however, the RPA amplification efficacy decreased when the temperature became higher than 42 ℃. Thus, the system reached its vertex at 43.3 °C, which was recognized as the optimal reaction temperature for the mcr-1 detection system. The fluorescence signal of the tet(X4) detection system gradually increased as the temperature increased from 37 to 42 ℃ and then decreased as the temperature increased from 42 to 45 ℃, indicating that 42 ℃ was the optimal temperature for the tet(X4) detection system (P < 0.05, ANOVA) (Fig. 3B). As the identified optimal temperature for the one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems (43.3 ℃ for mcr-1 and 42 ℃ for tet(X4)) differed from that for the RPA reactions in the aforementioned two-step detection system setup (39°C), the identified optimal primer pair sets for the two genes may not exhibit the best performance in the one-tube detection systems. Therefore, we evaluated the amplification efficiency of the three mcr-1 and tet(X4) RPA primer pairs in the one-tube detection systems. We found that mcr-RPA-3 and tet-RPA-1 remained the optimal primer pairs for the one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems (P < 0.05, ANOVA) (Fig. 3 C and D).
The optimal temperature determination (A and B), the optimal RPA primer set (C and D), and the optimal reaction time (E and F) for the one-tube RPA-CRISPR-Cas12b-based detection systems. Different letters on the top of columns denote significant differences (P < 0.05, ANOVA). Error bars represent means ± SEM (n = 3 replicates). The “background subtracted fluorescence” values were fluorescence values recorded in the reaction tubes minus the average fluorescence value obtained from three blank tubes
We then determined the optimal reaction time for the one-tube RPA-CRISPR-Cas12b-based detection systems. As depicted in Fig. 3E, the fluorescence signal intensity of the mcr-1 detection system gradually increased but became relatively stable from 45 min (P < 0.05, ANOVA). Hence, we determined that 45 min was the optimal reaction time for the one-tube mcr-1 detection system. The fluorescence signal of the tet(X4) detection system was no longer significantly enhanced after 30 min, indicating that the optimal reaction time for the tet(X4) detection system was 30 min (P < 0.05, ANOVA) (Fig. 3F).
Based on these optimized parameters, the one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems were developed (Fig. 4A).
The flowchart of the one-tube RPA-CRISPR-Cas12b-based detection system (A); the sensitivity assessment and fluorescence intensity of the one-tube RPA-CRISPR-Cas12b-based mcr-1 (B) and tet(X4) (C) detection system; the detection results of pork samples contaminated with different dose of mcr-1 (D) and tet(X4) (E) positive strain, PC: positive control, NC: negative control. Different letters on the top of columns denote significant differences (P < 0.05, ANOVA). Error bars represent means ± SEM (n = 3 replicates). The “background subtracted fluorescence” values were fluorescence values recorded in the reaction tubes minus the average fluorescence value obtained from three blank tubes
Assessment of sensitivity of one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems
We then assessed the sensitivity of the one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems using serially diluted DNA templates. A strong fluorescence signal was noted in tubes containing 6.25 × 108 to 6.25 × 100 copies of mcr-1 for the mcr-1 detection system; for the tet(X4) detection system, a strong fluorescence signal was observed in tubes containing 9 × 108 to 9 × 100 copies of tet(X4) (P < 0.05, ANOVA) (Fig. 4B and Fig. 4C). No obvious fluorescence signal was observed in tubes containing 6.25 × 10−1 copies of mcr-1 and 9 × 10−1 copies of tet(X4). The tubes containing 1.2 copies of mcr-1 and 1.5 copies of tet(X4) also did not exhibit strong fluorescence signals (Fig. S4). These results demonstrate that the capability of the one-tube RPA-CRISPR-Cas12b-based mcr-1 detection system to achieve real-time detection with a sensitivity as low as 6.25 copies of mcr-1. Additionally, the sensitivity of the tet(X4) detection system was observed to be nine copies of tet(X4).
Assessment of feasibility of one-tube RPA-CRISPR-Cas12b-based detection systems for onsite detection of mcr-1 and tet(X4).
The practicality of the one-tube RPA-CRISPR-Cas12b-based detection systems for the onsite detection of mcr-1 and tet(X4) was assessed using pork samples contaminated with E. coli DH5M containing the mcr-1-carrying pUC18 plasmid and E. coli DH5T containing the tet(X4)-carrying pUC18 plasmid. Crude DNA extracted from pork samples contaminated with 7 × 107 to 7 × 100 CFU/g of the mcr-1-positive strain E. coli DH5M (which was determined using the gradient dilution plating method) activated the mcr-1 detection system and produced a robust green fluorescence signal, suggesting that the detection system had a minimum detection limit of ~ 7 CFU/g of pork sample for the mcr-1-positive strain E. coli DH5M (Fig. 4D). Similarly, the feasibility of the one-tube RPA-CRISPR-Cas12b-based detection system for the onsite detection of tet(X4) was assessed using pork samples contaminated with tenfold gradient dilutions of E. coli DH5T. Strong green fluorescence signals were observed in tubes containing crude DNA extracted from pork samples contaminated with 7 × 107 to 7 × 100 CFU/g of tet(X4)-positive E. coli DH5T, indicating that the detection system had a minimum detection limit of ~ 7 CFU/g of pork sample for the tet(X4)-positive strain E. coli DH5T (Fig. 4E).
Finally, we used the one-tube RPA-CRISPR-Cas12b-based systems for detecting mcr-1 and tet(X4) contamination in pork products sold at retail markets and in environmental samples. We found that 15% (6/40) of the collected samples were mcr-1 positive, as revealed using the one-tube RPA-CRISPR-Cas12b-based mcr-1 detection system. However, only three out of the six positive samples were identified to be mcr-1 positive using the conventional cultivation-based (bacteria isolation followed by PCR amplification) method (Fig. S5). Notably, none of the samples found to be mcr-1 negative using the one-tube RPA-CRISPR-Cas12b-based detection system showed any positive result using the cultivation-based method. Furthermore, we performed a further bacterial enrichment experiment using the three samples that were identified to be mcr-1 positive using the RPA-CRISPR-Cas12b-based mcr-1 detection system but negative using the cultivation-based methods and obtained mcr-1-positive strains for two out of the three samples. For tet(X4) detection, all three methods revealed consistent results. Seven out of the 40 samples were identified to be tet(X4) positive (Fig. S6). These results collectively suggest that the sensitivity of the one-tube RPA-CRISPR-Cas12b-based detection systems developed in this study was higher than or equivalent to that of the conventional cultivation-based methods. However, our developed systems could produce results within 1 h, which is much shorter than the duration required by the cultivation-based (> 24 h) and PCR-based (> 2 h) methods.
We also selected an mcr-1-positive strain and a tet(X4)-positive strain isolated from the pork samples for whole-genome sequencing using Illumina short-read and Nanopore long-read technologies. The mcr-1-positive strain YZ3-11 was identified to be an E. fergusonii-affiliated strain. It was found to harbor five plasmids, and mcr-1 was located on a 66,073 bp plasmid designated pYZ3-11-mcr-1, which was found to be similar to the E. coli plasmid that was isolated from chickens in China (including pHNSD133-MCR (NCBI accession number MG725031.1), pWF-5-19C_mcr-1 (KX505142.1), pHLJ109-91 (MN232202.1)) and the E. coli plasmid that isolated from humans in China (including pmcr1_IncI2 (KU761326.1), pGZ49266 (MG210938.1), pPIB-3 (CP090405.1)). Y3-11-mcr-1 is classified as an IncI2-type plasmid, which has been demonstrated to be the preferred carrier for mcr-1 transmission among bacteria. The tet(X4)-positive strain YZ2-2 was identified to be a K. pneumoniae-affiliated strain. Three plasmids were identified in the YZ2-2 genome, and tet(X4) was located on a 90,338 bp plasmid designated pYZ2-2-tetX4, which was found to be similar to the K. aerogenes plasmids pNTT31XS-tetX4 (accession number, NZ_CP077430.1) isolated from porcine in China, the Klebsiella sp. plasmids pSDP9R-tetX4 (NZ_MW940621.1) isolated from pork in China, and the other five K. pneumoniae strains that isolated from humans in China (including pUnnamed2 (CP107293.1), pUnnamed2 (CP107299.1), pS234-2 (CP102188.1), pJX7-2 (CP064225.1), pJX8-2 (CP064219.1)) (Fig. 5B). The pYZ2-2-tetX4 plasmid is classified as an IncFII-type plasmid, which could contribute to form multiple-replicon plasmids via fusion of different single replicons, such as IncFIB, IncI1 and IncQ1, thus significantly increasing the risk of multi-drug resistance transmission (Li et al. 2021; Wang et al. 2021). In short, these results collectively indicated that there is a great risk of transmission of mcr-1 and tet(X4) between humans, animals and their corresponding meat products.
Discussion
Colistin and tigecycline are recognized as the “last line of defense” antibiotics for the treatment of infections caused by multidrug-resistant gram-negative bacteria in clinics (Lu et al. 2021; Osei et al., 2016); however, the recent emergence and quick spread of plasmid-mediated mcr and tet(X) have dramatically affected the established antibiotic therapy regimens, which could result in a lack of effective antibiotics for treating infectious diseases and compromise modern clinical medicine. Among the identified mcr and tet(X) variants, mcr-1 and tet(X4) are recognized as important and prevalent gene types for colistin and tigecycline resistance, respectively (Dadashi et al. 2022; He et al. 2019). The rapid and accurate detection of mcr-1 and tet(X4) is crucial for ensuring the success of antibiotic therapy in clinics and for controlling the spread and transmission of mcr-1 and tet(X4) in animals and humans. In this study, we developed RPA-CRISPR-Cas-based nucleic acid detection systems for the rapid onsite detection of mcr-1 and tet(X4).
The CRISPR-Cas-based nucleic acid detection system usually exhibits high specificity and sensitivity, and sgRNA is the crucial component of the detection system that guarantees its specificity (Li et al. 2022a, b, c, d, e). Therefore, the identification of a target-conserved and -specific protospacer sequence, which is the basis of sgRNA, is the crucial step for the assembly of CRISPR-Cas-based detection systems. In this study, we identified mcr-1- and tet(X4)-conserved and -specific protospacer sequences for assembling CRISPR-Cas12b-based detection systems using a comprehensive BLAST approach. The identified protospacers were highly conserved in mcr-1 and tet(X4) but were only present in several irrelevant eukaryotic genomes based on the comprehensive NCBI nt database, which contains 91,700,559 sequences. The assembled CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems were proven to be activated only by the mcr-1-positive and tet(X4)-positive samples, respectively (Fig. 2 and S4), further demonstrating the efficacy of our bioinformatics-guided protospacer identification approach.
Notably, the CRISPR-Cas system can be combined with isothermal amplification methods, such as RPA, which offer the advantages of simplicity and rapidity, to further enhance the specificity and sensitivity of detection (Hijjawi et al. 2023; Li et al. 2022a, b, c, d, e). However, this approach involves nucleic acid amplification before detection using the CRISPR-Cas system, which may result in aerosol contamination (Lin et al. 2022). Moreover, this stepwise approach lacks convenience for onsite detection purposes. This problem can be effectively overcome by integrating nucleic acid amplification and CRISPR-Cas detection in one tube without opening the lid during the process (Lin et al. 2022). Based on this principle, we developed one-tube RPA-CRISPR-Cas12b-based detection systems that could detect as low as 6.25 copies/µL of mcr-1 and nine copies/µL of tet(X4) within an hour. The conventional cultivation- and PCR-based mcr-1 and tet(X4) detection methods required a sample-to-answer turnover time of > 24 h and ~ 2 h, respectively. Therefore, our developed one-tube RPA-CRISPR-Cas12b-based detection systems provided a significant advantage in terms of detection time. Furthermore, compared with other ARG detection methods, the one-tube RPA-CRISPR-Cas12b-based method exhibited a certain advantage in terms of sensitivity as well. For example, the detection levels of mcr-1 and tet(X4) based on fluorescence quantitative assays were 10 copies and 102 copies, respectively (Donà et al. 2017; Li et al. 2020). We then performed mcr-1 and tet(X4) detection simultaneously using our developed RPA-CRISPR-Cas12b-based detection systems and conventional cultivation-based methods to assess the prevalence of mcr-1 and tet(X4) in retail pork samples and associated environmental samples. The one-tube RPA-CRISPR-Cas12b-based detection systems identified a total of six mcr-1-positive samples, while the conventional cultivation-based methods detected three of the six positive samples (Fig. S4). After further bacterial enrichment in the samples that were identified to be mcr-1 positive using the one-tube RPA-CRISPR-Cas12b-based detection systems but negative using the cultivation-based method, mcr-1-positive strains were successfully isolated from two out of the three samples, and no mcr-1-positive strain was detected from sample 28 after screening 50 colonies using the colony PCR method. This discrepancy could suggest a notably lower population of mcr-1-positive strains in sample 28, possibly requiring a larger number of colonies for positive identification. While our extensive blast analysis indicated a low likelihood of false positives, the possibility of a false positive cannot be completely ruled out. Nevertheless, the one-tube RPA-CRISPR-Cas12b-based detection systems exhibited higher sensitivity compared with the cultivation-based method, and exhibited great potential to guide the mcr-1- and tet(X4)-positive strain isolation. However, to validate the sensitivity and specificity of the one-tube RPA-CRISPR-Cas12b-based detection systems, further large-scale sampling and detection are imperative in future studies. In addition, more analysis, encompassing the design of different mcr-1 and tet(X4) sgRNAs along with exploration of varied reaction conditions, can be undertaken to enhance sensitivity and specificity while reducing the sample-to-answer time.
In addition, the high prevalence of mcr-1 and tet(X4) in the pork samples and associated environmental samples revealed using the detection systems suggested that pork serves as a significant reservoir of mcr-1 and tet(X4); these results are consistent with previous findings. For example, in a study on E. coli isolated from pork samples in Thailand, the prevalence of mcr-1-positive strains was as high as 56.1% (Pungpian et al. 2021). Similarly, Li et al. isolated and identified 23 tet(X4)-positive strains from 53 pork samples collected from markets in Jiangsu, China, and found that these strains were resistant to multiple antibiotics (Li et al. 2022a, b, c, d, e). One mcr-1-positive strain (YZ3-11) and one tet(X4)-positive strain (YZ2-2) were selected for whole-genome sequencing in our study. We found that mcr-1 was located on the IncI2-type plasmid, which shared high similarity with the chicken-derived and human-derived plasmid isolated from China. Interestingly, this plasmid harbored only one resistance gene mcr-1. Moreover, tet(X4) was located on the IncFII-type plasmid, which exhibited high similarity with the plasmid isolated from porcine, pork, and humans in China. This result suggests that mcr-1 and tet(X4) can be transmitted between humans, animals, and their corresponding meat products through the food chain.
Overall, a rapid and accurate one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems were developed, which exhibited exceptional specificity and sensitivity with a relatively short detection time (~ 55 min and ~ 40 min for mcr-1 and tet(X4) detection, respectively). The detection systems had a detection sensitivity of 6.25 copies for mcr-1 and nine copies for tet(X4). Moreover, their minimum detection limit was 7 CFU/g of pork sample for both mcr-1- and tet(X4)-positive strains. Moreover, we validated the efficacy of the detection systems using pork samples and environmental samples collected from retail markets and confirmed their application potential for the onsite detection of mcr-1 and tet(X4). Overall, our developed rapid and accurate one-tube RPA-CRISPR-Cas12b-based mcr-1 and tet(X4) detection systems are simple and contamination-free, require inexpensive instrumentation, and produce results within 1 h. These systems could be potentially applied to the surveillance of ARGs in the future.
Data availability
The BioProject accession number of YZ3-11 was PRJNA979119, and the accession numbers for the sequences were CP127003- CP127008; The BioProject accession number of YZ2-2 was PRJNA979126, and the accession numbers for the sequences were CP126999- CP127003.
References
Alcock BP, Raphenya AR, Lau TT, Tsang KK, Bouchard M, Edalatmand A, Huynh W, Nguyen ALV, Cheng AA, Liu S (2020) CARD 2020: antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res 48(D1):D517–D525. https://doi.org/10.1093/nar/gkz935
Alikhan NF, Petty NK, Ben Zakour NL, Beatson SA (2011) BLAST Ring Image Generator (BRIG): simple prokaryote genome comparisons. BMC Genom 12(1):1–10. https://doi.org/10.1186/1471-2164-12-402
Aman R, Marsic T, Sivakrishna Rao G, Mahas A, Ali Z, Alsanea M, Al-Qahtani A, Alhamlan F, Mahfouz M (2022) iSCAN-V2: A one-pot RT-RPA–CRISPR/Cas12b assay for point-of-care SARS-CoV-2 detection. Front Bioeng Biotechnol 21(9):800104. https://doi.org/10.3389/fbioe.2021.800104
Carattoli A, Zankari E, García-Fernández A, Voldby Larsen M, Lund O, Villa L, Møller Aarestrup F, Hasman H (2014) In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 58(7):3895–3903. https://doi.org/10.1128/AAC.02412-14
Chabou S, Leangapichart T, Okdah L, Le Page S, Hadjadj L, Rolain JM (2016) Real-time quantitative PCR assay with Taqman® probe for rapid detection of MCR-1 plasmid-mediated colistin resistance. New Microbes New Infect 13:71–74. https://doi.org/10.1016/j.nmni.2016.06.017
Che YL, Wu RJ, Li HJ, Wang LB, Wu XM, Chen QY, Chen RJ, Zhou LJ (2023) Characterization of two novel colistin resistance gene mcr-1 variants originated from Moraxella spp. Front Microbiol 14:1153740. https://doi.org/10.3389/fmicb.2023.1153740
Coppi M, Cannatelli A, Antonelli A, Baccani I, Di Pilato V, Sennati S, Giani T, Rossolini GM (2018) A simple phenotypic method for screening of MCR-1-mediated colistin resistance. Clin Microbiol Infect 24(2):e201-203. https://doi.org/10.1016/j.cmi.2017.08.011
Cui CY, Chen C, Liu BT, He Q, Wu XT, Sun RY, Zhang Y, Cui ZH, Guo WY, Ql Jia (2020) Co-occurrence of plasmid-mediated tigecycline and carbapenem resistance in Acinetobacter spp. from waterfowls and their neighboring environment. Antimicrob Agents Chemother 64(5):e02502-02519. https://doi.org/10.1128/AAC.02502-19
Cui CY, Li XJ, Chen C, Wu XT, He Q, Jia QL, Zhang XJ, Lin ZY, Li C, Fang LX (2022) Comprehensive analysis of plasmid-mediated tet(X4)-positive Escherichia coli isolates from clinical settings revealed a high correlation with animals and environments-derived strains. Sci Total Environ 806:150687. https://doi.org/10.1016/j.scitotenv.2021.150687
Dadashi M, Sameni F, Bostanshirin N, Yaslianifard S, Khosravi-Dehaghi N, Nasiri MJ, Goudarzi M, Hashemi A, Hajikhani B (2022) Global prevalence and molecular epidemiology of mcr-mediated colistin resistance in Escherichia coli clinical isolates: A systematic review. J Glob Antimicrob Resist 29:444–461. https://doi.org/10.1016/j.jgar.2021.10.022
de Mesquita Souza Saraiva M, Lim K, do Monte DFM, Givisiez P EN, Alves LBR., de Freitas Neto OC, Kariuki S, Júnior AB, de Oliveira CJB, Gebreyes WA (2022) Antimicrobial resistance in the globalized food chain: A One Health perspective applied to the poultry industry. Braz J Microbiol 53(1):465-486https://doi.org/10.1007/s42770-021-00635-8
Donà V, Bernasconi OJ, Kasraian S, Tinguely R, Endimiani A (2017) A SYBR® Green-based real-time PCR method for improved detection of mcr-1-mediated colistin resistance in human stool samples. J Glob Antimicrob Resist 9:57–60. https://doi.org/10.1016/j.jgar.2017.01.007
Doumith M, Godbole G, Ashton P, Larkin L, Dallman T, Day M, Day M, Muller-Pebody B, Ellington MJ, de Pinna E (2016) Detection of the plasmid-mediated mcr-1 gene conferring colistin resistance in human and food isolates of Salmonella enterica and Escherichia coli in England and Wales. J Antimicrob Chemother 71(8):2300–2305. https://doi.org/10.1093/jac/dkw093
Exner M, Bhattacharya S, Christiansen B, Gebel J, Goroncy-Bermes P, Hartemann P, Heeg P, Ilschner C, Kramer A, Larson E (2017) Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg Infect Contr 12:Doc05. https://doi.org/10.3205/dgkh000290
He T, Wang R, Liu D, Walsh TR, Zhang R, Lv Y, Ke Y, Ji Q, Wei R, Liu Z (2019) Emergence of plasmid-mediated high-level tigecycline resistance genes in animals and humans. Nat Microbiol 4(9):1450–1456. https://doi.org/10.1038/s41564-019-0445-2
Hijjawi N, Zahedi A, Ryan U (2023) Point of care diagnostics for Cryptosporidium: new and emerging technologies. Curr Opin Gastroenterol 39(1):3–8. https://doi.org/10.1097/MOG.0000000000000895
Hu J, Fan J, Sun Z, Liu S (2020) NextPolish: a fast and efficient genome polishing tool for long-read assembly. Bioinformatics 36(7):2253–2255. https://doi.org/10.1093/bioinformatics/btz891
Hu F, Liu Y, Zhao S, Zhang Z, Li X, Peng N, Jiang Z (2022) A one-pot CRISPR/Cas13a-based contamination-free biosensor for low-cost and rapid nucleic acid diagnostics. Biosens Bioelectron 202:113994. https://doi.org/10.1016/j.bios.2022.113994
Huang Y, Gu D, Xue H, Yu JY, Tang YY, Huang JL, Zhang YZ, Jiao XA (2021) Rapid and accurate Campylobacter jejuni detection with CRISPR-Cas12b based on newly identified Campylobacter jejuni-specific and-conserved genomic signatures. Front Microbiol 12:649010. https://doi.org/10.3389/fmicb.2021.649010
Kolmogorov M, Yuan J, Lin Y, Pevzner PA (2019) Assembly of long, error-prone reads using repeat graphs. Nat Biotechnol 37(5):540–546. https://doi.org/10.1038/s41587-019-0072-8
Li Y, Li S, Wang J, Liu G (2019) CRISPR/Cas systems towards next-generation biosensing. Trends Biotechnol 37(7):730–743. https://doi.org/10.1016/j.tibtech.2018.12.005
Li Y, Shen Z, Ding S, Wang S (2020) A TaqMan-based multiplex real-time PCR assay for the rapid detection of tigecycline resistance genes from bacteria, faeces and environmental samples. BMC Microbiol 20:1–7. https://doi.org/10.1186/s12866-020-01813-8
Li Y, Wang Q, Peng K, Liu Y, Xiao X, Mohsin M, Li R, Wang Z (2021) Distribution and genomic characterization of tigecycline-resistant tet(X4)-positive Escherichia coli of swine farm origin. Microb Genom 7(10):000667. https://doi.org/10.1099/mgen.0.000667
Li F, Xiao J, Yang H, Yao Y, Li J, Zheng H, Guo Q, Wang X, Chen Y, Guo Y (2022a) Development of a rapid and efficient RPA-CRISPR/Cas12a assay for Mycoplasma pneumoniae detection. Front Microbiol 13:858806. https://doi.org/10.3389/fmicb.2022.858806
Li R, Lu X, Munir A, Abdullah S, Liu Y, Xiao X, Wang Z, Mohsin M (2022b) Widespread prevalence and molecular epidemiology of tet(X4) and mcr-1 harboring Escherichia coli isolated from chickens in Pakistan. Sci Total Environ 806:150689. https://doi.org/10.1016/j.scitotenv.2021.150689
Li Y, Li Y, Bu K, Wang M, Wang Z, Li R (2022c) Antimicrobial resistance and genomic epidemiology of tet(X4)-bearing bacteria of pork origin in Jiangsu. China Genes 14(1):36. https://doi.org/10.3390/genes14010036
Li Y, Man S, Ye S, Liu G, Ma L (2022d) CRISPR-Cas-based detection for food safety problems: Current status, challenges, and opportunities. Compr Rev Food Sci Food Saf 21(4):3770–3798. https://doi.org/10.1111/1541-4337.13000
Li Y, Shi Z, Hu A, Cui J, Yang K, Liu Y, Deng G, Zhu C, Zhu L (2022e) Rapid one-tube RPA-CRISPR/Cas12 detection platform for methicillin-resistant Staphylococcus aureus. Diagnostics 12(4):829. https://doi.org/10.3390/diagnostics12040829
Li XP, Zhong JY, Li HY, Qiao YB, Mao XL, Fan HY, Zhong YW, Imani S, Zheng SS, Li JH (2023) Advances in the application of CRISPR-Cas technology in rapid detection of pathogen nucleic acid. Front Mol Biosci 10:260883. https://doi.org/10.3389/fmolb.2023.1260883
Lin M, Yue H, Tian T, Xiong E, Zhu D, Jiang Y, Zhou X (2022) Glycerol additive boosts 100-fold sensitivity enhancement for one-pot RPA-CRISPR/Cas12a assay. Anal Chem 94(23):8277–8284. https://doi.org/10.1021/acs.analchem.2c00616
Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian G, Dong B, Huang X (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16(2):161–168. https://doi.org/10.1016/s1473-3099(15)00424-7
Lu X, Xiao X, Liu Y, Li R, Wang Z (2021) Emerging opportunity and destiny of mcr-1-and tet(X4)-coharboring plasmids in Escherichia coli. Microbiol Spectr 9(3):e01520-01521. https://doi.org/10.1128/spectrum.01520-21
Manyi-Loh C, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23(4):795. https://doi.org/10.3390/molecules23040795
VT Nair D, Venkitanarayanan K, Kollanoor Johny A (2018) Antibiotic-resistant Salmonella in the food supply and the potential role of antibiotic alternatives for control. Foods 7(10):167. https://doi.org/10.3390/foods7100167
Osei Sekyere J, Govinden U, Bester L, Essack S (2016) Colistin and tigecycline resistance in carbapenemase-producing Gram-negative bacteria: emerging resistance mechanisms and detection methods. J Appl Microbiol 121(3):601–617. https://doi.org/10.1111/jam.13169
Pungpian C, Lee S, Trongjit S, Sinwat N, Angkititrakul S, Prathan R, Srisanga S, Chuanchuen R (2021) Colistin resistance and plasmid-mediated mcr genes in Escherichia coli and Salmonella isolated from pigs, pig carcass and pork in Thailand, Lao PDR and Cambodia border provinces. J Vet Sci 22(5):e68. https://doi.org/10.4142/jvs.2021.22.e68
Qiu XT, Liu XP, Ma X, Wang RX, Chen SL, Li F, Li ZJ (2022) One-pot isothermal LAMP-CRISPR-based assay for Klebsiella pneumoniae detection. Microbiol Spectr 10(4):e01545-e1622. https://doi.org/10.1128/spectrum.01545-22
Qiu XT, Liu X, Wang RX, Ma X, Han LC, Yao J, Li ZJ (2023) Accurate, sensitive, and rapid detection of Pseudomonas aeruginosa based on CRISPR/Cas12b with one fluid-handling step. Microbiol Spectr 11(1):e03523 22. https://doi.org/10.1128/spectrum.03523-22
Serwecińska L (2020) Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water 12(12):3313. https://doi.org/10.3390/w12123313
Shanmugakani RK, Akeda Y, Sugawara Y, Laolerd W, Chaihongsa N, Sirichot S, Yamamoto N, Hagiya H, Morii D, Fujiya Y (2019) PCR-dipstick-oriented surveillance and characterization of mcr-1-and carbapenemase-carrying Enterobacteriaceae in a Thai hospital. Front Microbiol 10:149. https://doi.org/10.3389/fmicb.2019.00149
Sun H, Wan Y, Du P, Liu D, Li R, Zhang P, Wu Y, Fanning S, Wang Y, Bai L (2021) Investigation of tigecycline resistant Escherichia coli from raw meat reveals potential transmission among food-producing animals. Food Control 121:107633. https://doi.org/10.1016/j.foodcont.2020.107633
Teillant A, Laxminarayan R (2015) Economics of antibiotic use in US swine and poultry production. Choices 30(1):1–11. https://doi.org/10.1128/spectrum.00495-21
Varshney RK, Barmukh R, Roorkiwal M, Qi Y, Kholova J, Tuberosa R, Reynolds MP, Tardieu F, Siddique KH (2021) Breeding custom-designed crops for improved drought adaptation. Adv Genet 2(3):e202100017. https://doi.org/10.1002/ggn2.202100017
Wang X, Zhao J, Ji F, Chang H, Qin J, Zhang C, Hu G, Zhu J, Yang J, Jia Z (2021) Multiple-replicon resistance plasmids of Klebsiella mediate extensive dissemination of antimicrobial genes. Front Microbiol 12:754931. https://doi.org/10.3389/fmicb.2021.754931
Wang H, Jia CH, Li HZ, Yin R, Chen J, Li Y, Yue M (2022a) Paving the way for precise diagnostics of antimicrobial resistant bacteria. Front Mol Biosci 9:976705. https://doi.org/10.3389/fmolb.2022.976705
Wang Q, Lei C, Cheng H, Yang X, Huang Z, Chen X, Ju Z, Zhang H, Wang H (2022b) Widespread dissemination of plasmid-mediated tigecycline resistance gene tet(X4) in Enterobacterales of porcine origin. Microbiol Spectr 10(5):e01615-01622. https://doi.org/10.1128/spectrum.01615-22
Woolhouse ME, Ward MJ (2013) Sources of antimicrobial resistance. Science 341(6153):1460–1461
Yang H, Gao P, Rajashankar KR, Patel DJ (2016) PAM-dependent target DNA recognition and cleavage by C2c1 CRISPR-Cas endonuclease. Cell 167(7):1814–1828. https://doi.org/10.1016/j.cell.2016.11.053
Yang XG, Huang JF, Chen YJ, Ying X, Tan QQ, Chen X, Zeng XY, Lei SG, Wang Y, Li SJ (2023) Development of CRISPR/Cas12b-based multiple cross displacement amplification technique for the detection of mycobacterium tuberculosis complex in clinical settings. Microbiol Spectr 11(2):e03475-e3522. https://doi.org/10.1128/spectrum.03475-22
Zhang Z, Zhan Z, Shi C (2022b) International spread of Tet(X4)-producing Escherichia coli isolates. Foods 11(14):2010. https://doi.org/10.3390/foods11142010
Zhang S, Wen J, Wang Y, Wang M, Jia R, Chen S, Liu M, Zhu D, Zhao X, Wu Y (2022) Dissemination and prevalence of plasmid-mediated high-level tigecycline resistance gene tet(X4). Front Microbiol 13:969769. https://doi.org/10.3389/fmicb.2022.969769
Zhao ZY, Lu MH, Wang N, Li YR, Zhao LJ, Zhang Q, M SL, Ye SY, Ma L (2023) Nanomaterials-assisted CRISPR/Cas detection for food safety: Advances, challenges and future prospects. Trends Analyt Chem 16: 117269. https://doi.org/10.1016/j.trac.2023.117269
Zhu YG, Zhao Y, Li B, Huang CL, Zhang SY, Yu S, Chen YS, Zhang T, Gillings MR, Su JQ (2017) Continental-scale pollution of estuaries with antibiotic resistance genes. Nat Microbiol 2:16270. https://doi.org/10.1038/nmicrobiol.2016.270
Funding
This work was financially supported by the 111 Project (grant number D18007) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (grant number PAPD).
Author information
Authors and Affiliations
Contributions
YZZ and YW conceived and revised the manuscript. HC and QYP conducted the experiments and collected the data. XAJ and YZZ contributed funding acquisition. All authors have read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, Y., Chen, H., Pan, Q. et al. Development and evaluation of rapid and accurate one-tube RPA-CRISPR-Cas12b-based detection of mcr-1 and tet(X4). Appl Microbiol Biotechnol 108, 345 (2024). https://doi.org/10.1007/s00253-024-13191-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13191-6