Abstract
The human microbiome, a diverse ecosystem of microorganisms within the body, plays pivotal roles in health and disease. This review explores site-specific microbiomes, their role in maintaining health, and strategies for their upkeep, focusing on oral, lung, vaginal, skin, and gut microbiota, and their systemic connections. Understanding the intricate relationships between these microbial communities is crucial for unraveling mechanisms underlying human health. Recent research highlights bidirectional communication between the gut and distant microbiome sites, influencing immune function, metabolism, and disease susceptibility. Alterations in one microbiome can impact others, emphasizing their interconnectedness and collective influence on human physiology. The therapeutic potential of gut microbiota in modulating distant microbiomes offers promising avenues for interventions targeting various disorders. Through interdisciplinary collaboration and technological advancements, we can harness the power of the microbiome to revolutionize healthcare, emphasizing microbiome-centric approaches to promote holistic well-being while identifying areas for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hippocrates is thought to have said that “all disease begins in the gut.” While the human body is host to a complex and diverse community of microorganisms, collectively known as the microbiome, which inhabits various niches within the body, the gut houses the largest number of microbes (Dekaboruah et al. 2020). These microbial ecosystems play fundamental roles in maintaining health and influencing disease processes (Hou et al. 2022). Understanding the intricacies of the human microbiome and its site-specific dynamics is crucial for unraveling the mechanisms underlying human health and disease. By exploring the interconnectedness of these microbial communities and their systemic connections with the host, we aim to shed light on how alterations in one microbiome can impact others, shaping human physiology and disease susceptibility (Kho and Lal 2018). Recent research has uncovered bidirectional communication between the gut microbiota and distant microbiome sites, influencing immune function, metabolism, and disease progression (Carabotti et al. 2015; Enaud et al. 2020; Park et al. 2021; Thye et al. 2022) (Fig. 1). Moreover, emerging evidence suggests that therapeutic interventions targeting the microbiome hold promise for modulating distant microbiomes and treating a range of disorders, including oral infections, respiratory diseases, vaginal dysbiosis, and dermatological conditions (Amabebe and Anumba 2020; Hou et al. 2022; Du et al. 2022).
Through interdisciplinary collaboration and technological advancements, researchers are poised to harness the therapeutic potential of the microbiome to revolutionize healthcare (Liwinski and Elinav 2020). By developing personalized treatments that target the gut microbiome, we can aspire to improve patient outcomes and promote holistic health and well-being. This review aims to provide comprehensive understanding of the human microbiome and how the gut microbes interact with other microbes found in different parts of the body. It also points out areas where more research is needed, highlighting how focusing on the microbiome can help us understand and tackle health issues.
Oral microbiome
The oral microbiome refers to the diverse community of microorganisms residing within the oral cavity, including bacteria, viruses, fungi, and archaea (Li et al. 2022a, 2022b). This complex ecosystem plays a pivotal role in maintaining oral health by contributing to various physiological processes such as saliva metabolism, immune modulation, and dental biofilm formation (Kilian et al. 2016). Comprising hundreds of bacterial species, the oral microbiome exists in a dynamic balance, with interactions between different microbial taxa influencing its composition and function (Dewhirst et al. 2010). While some oral bacteria are considered beneficial and contribute to oral homeostasis, others may become pathogenic under certain conditions, leading to the development of oral diseases such as periodontitis and dental caries (Lamont et al. 2018). Additionally, research has elucidated the bidirectional relationship between the oral microbiome and systemic health, with dysbiosis in the oral microbiota implicated in various systemic conditions including cardiovascular diseases, diabetes mellitus, and adverse pregnancy outcomes (Tonetti et al. 2013; Ye and Kapila 2021; Qin et al. 2022). Therefore, understanding the composition and function of the oral microbiome is essential for comprehending its role in both oral and systemic health.
Dental health: how the microbiota affects oral hygiene
Dental health is intricately linked to the composition and balance of the oral microbiota, the diverse community of microorganisms inhabiting the oral cavity (Dewhirst et al. 2010). One key aspect of the oral microbiota influence on dental health is its role in the formation and maintenance of dental biofilms, commonly known as dental plaque. Dental plaque is a structured microbial community that adheres to tooth surfaces and is primarily composed of bacteria embedded in a matrix of extracellular polymeric substances (Marsh 2003). The microbial composition of dental plaque varies depending on factors such as oral hygiene practices, diet, and host immune responses (Marsh 2003). Certain bacterial species within dental plaque, such as Streptococcus mutans and Lactobacillus species, are known to be associated with dental caries, the most prevalent oral disease worldwide (Ahmed et al. 2014). Furthermore, the oral microbiota plays a crucial role in modulating the host immune response within the oral cavity. The interaction between oral bacteria and the host immune system can have both beneficial and detrimental effects on oral health. For instance, commensal bacteria in the oral microbiota contribute to the development and maintenance of oral mucosal immunity, protecting the host from pathogenic invaders (Yu et al. 2019). On the other hand, dysbiosis in the oral microbiota, characterized by shifts in microbial composition and function, can lead to chronic inflammatory conditions such as periodontitis, a common oral disease characterized by destruction of the tooth-supporting tissues (Genco and Borgnakke 2013).
Understanding the intricate relationship between the oral microbiota and dental health is essential for developing effective preventive and therapeutic strategies. By targeting specific components of the oral microbiota associated with oral diseases, such as caries and periodontitis, novel approaches for disease prevention and treatment can be developed. These may include interventions aimed at modulating the oral microbiota through probiotics, prebiotics, or antimicrobial agents, as well as personalized oral hygiene regimens tailored to an individual’s oral microbial profile (Marsh 2003; Haukioja 2010). Further research into the complex interactions between the oral microbiota and host factors is necessary to develop targeted interventions for maintaining oral health and preventing oral diseases.
Oral-systemic connection: exploring the link between oral microbiome and overall health
Research has revealed intricate connections between the oral microbiome and various systemic conditions, highlighting the importance of oral health maintenance for overall well-being (Tonetti et al. 2013; Kato et al. 2018; Gao et al. 2018). One key aspect of the oral-systemic connection is the association between periodontal disease and systemic inflammatory conditions such as cardiovascular diseases, inflammatory bowel disease, and certain type of cancers (Tonetti et al. 2013; Kato et al. 2018; Zhong et al. 2021; Wang et al. 2021a, 2021b). Periodontal disease, characterized by chronic inflammation and destruction of the tooth-supporting tissues, is often driven by dysbiosis in the oral microbiota (Darveau 2010). Oral pathogens and their byproducts can enter the bloodstream through inflamed periodontal tissues, leading to systemic dissemination and contributing to the development and progression of systemic diseases (Darveau 2010).
Moreover, the oral microbiome has been implicated in the modulation of immune responses and systemic inflammation, further linking oral health to systemic health outcomes (Tonetti et al. 2013; Gao et al. 2018; Ye and Kapila 2021; Qin et al. 2022). Dysbiosis in the oral microbiota can lead to increased production of pro-inflammatory cytokines and activation of systemic immune responses, which may contribute to the pathogenesis of systemic diseases (Darveau 2010; Hajishengallis 2015). Understanding the link between the oral microbiome and systemic health is essential for developing preventive and therapeutic strategies to mitigate the risk of systemic diseases associated with oral dysbiosis. By promoting oral health and targeting dysbiotic changes in the oral microbiome, interventions aimed at reducing the risk of systemic diseases can be developed (Darveau 2010; Peng et al. 2022). In conclusion, the oral microbiome plays a crucial role in the oral-systemic connection, influencing systemic health outcomes through its involvement in periodontal disease, immune modulation, and systemic inflammation. More focused research into the mechanisms underlying the oral-systemic connection is necessary to develop effective strategies for maintaining both oral and systemic health
Probiotic solutions: balancing the oral microbiome for improved health
Probiotics, defined as live microorganisms that confer health benefits when administered in adequate amounts, have emerged as promising agents for balancing the oral microbiome and promoting oral health (Haukioja 2010; Hill et al. 2014). These beneficial microorganisms can exert their effects by competing with and inhibiting the growth of pathogenic bacteria, modulating host immune responses, and enhancing the integrity of the oral epithelial barrier (Hill et al. 2014). Several strains of probiotic bacteria, such as Lactobacillus and Bifidobacterium species, have been investigated for their potential to improve oral health outcomes (Teughels et al. 2011). In a recent study conducted on teenagers, they received Lactobacillus reuteri DSM 17938/ATCC 5289 for 28 days, showing a less pronounced increase in Streptococcus mutans levels along with improvements in plaque, gingivitis, and bleeding (Borrell Garcia et al. 2021). Clinical studies have demonstrated that oral administration of probiotics can lead to a reduction in oral microbial counts, decreased plaque accumulation, and improvements in parameters such as gingival health and breath odor (Teughels et al. 2011; Zhang et al. 2022). Probiotics that are beneficial for oral health predominantly belong to genera such as Lactobacillus, Bifidobacterium, Streptococcus, and Weissella, alongside selected species like Bacillus subtilis and Saccharomyces cerevisiae. Notably, various strains of Lactobacillus reuteri, Lactobacillus brevis, and Streptococcus salivarius, among others, have been commercialized as probiotics to promote oral health. These probiotic strains are typically sourced from microorganisms isolated from the oral cavity (Allaker and Stephen 2017; Mahasneh and Mahasneh 2017).
Moreover, probiotics have been shown to have systemic effects beyond the oral cavity, including modulation of systemic immune responses and reduction of systemic inflammation (Suez et al. 2019). By promoting a balanced and healthy oral microbiome, probiotics may contribute to the prevention and management of systemic diseases associated with oral dysbiosis, such as cardiovascular diseases, diabetes mellitus, etc. (Suez et al. 2019). Overall, probiotics offer a promising approach for balancing the oral microbiome and improving oral health outcomes. Further research is needed to elucidate the specific mechanisms of action of probiotics in the oral cavity and to identify the most effective strains and formulations for oral health promotion.
Gut connection: investigating the relationship between oral microbiome and gut health
The oral cavity which serves as a reservoir for diverse microbial populations, and disturbances in oral microbiota composition can have downstream effects on gut microbial ecology (Segata et al. 2012; Elzayat et al. 2023). Studies have shown that oral bacteria in mice can translocate to the gastrointestinal tract through swallowing, influencing the microbial composition and function of the gut microbiome (Atarashi et al. 2017). Furthermore, dysbiosis in the oral microbiome has been associated with gastrointestinal disorders such as inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and colorectal cancer (Kato et al. 2018; Zhong et al. 2021; Wang et al. 2021a, 2021b). Pathogens found in the oral cavity, along with their metabolic byproducts, have the potential to initiate systemic immune reactions and inflammation, which may worsen gut inflammation and play a role in the development of gastrointestinal diseases (Peng et al. 2022). Conversely, interventions aimed at promoting oral health, such as probiotic supplementation and improved oral hygiene practices, may have beneficial effects on gut health. Probiotics have been shown to modulate gut microbial composition and improve gastrointestinal symptoms in certain conditions (Allaker and Stephen 2017; Mahasneh and Mahasneh 2017; Suez et al. 2019).
Smoking also significantly influences the oral microbiome, resulting in dysbiosis characterized by decreased microbial diversity and increased abundance of pathogenic species. This imbalance is associated with various oral health issues, including periodontal disease, dental caries, and oral cancer. Studies have shown that smoking promotes the growth of harmful bacteria such as Porphyromonas gingivalis and Tannerella forsythia, while reducing beneficial species like Streptococcus salivarius and Lactobacillus species. Consequently, smoking disrupts the natural balance of the oral microbial ecosystem (Wu et al. 2016; Nagakubo and Kaibori 2023).
Lung microbiome
The lung microbiome once thought to be sterile is now recognized as a diverse microbial community residing within the lower respiratory tract. Comprising bacteria, fungi, viruses, and archaea, the lung microbiome plays a crucial role in maintaining respiratory health and homeostasis (Dickson et al. 2013). Dysbiosis in the lung microbiome has been associated with respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), and cystic fibrosis (Erb-Downward et al. 2011; Dickson et al. 2013; Garcia-Nunez et al. 2014; Campbell et al. 2023). Furthermore, the lung microbiome interacts with the host immune system, modulating immune responses and inflammation within the respiratory tract. Dysregulated immune-microbial interactions can contribute to the pathogenesis of respiratory diseases and exacerbate disease severity (Huffnagle et al. 2017).
Understanding the composition and function of the lung microbiome is essential for elucidating its role in respiratory health and disease. Targeted interventions aimed at modulating the lung microbiome, such as probiotics, antimicrobial agents, and microbiota transplantation, hold promise for improving respiratory outcomes and managing lung diseases (Cookson et al. 2018; Li et al. 2022a, 2022b; Yuksel et al. 2023). Overall, the lung microbiome is a dynamic microbial ecosystem that influences respiratory health through interactions with the host immune system. More comprehensive research into the composition, function, and impact of lung microbiome on respiratory diseases is necessary to develop novel therapeutic strategies and improve patient outcomes.
Respiratory health: unraveling the importance of the lung microbiome
Exploring the lung microbiome unveils its crucial role in respiratory health. Recent advancements in sequencing technologies have enabled the identification of diverse microbial communities inhabiting the lower respiratory tract collectively called as lung microbiome. While the lung microbiome in healthy individuals is characterized by low microbial biomass and high diversity, dysbiosis in this ecosystem has been linked to various respiratory diseases, including asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis and many more (Erb-Downward et al. 2011; Dickson et al. 2013; Garcia-Nunez et al. 2014; Campbell et al. 2023).
Several key bacterial species have been identified as important contributors to respiratory health. For instance, studies have shown that the presence of commensal bacteria such as Streptococcus, Prevotella, and Veillonella in the lung microbiome is associated with improved lung function and reduced risk of respiratory infections (Segal et al. 2016). In contrast, dysbiosis of microbes is characterized by an overabundance of pathogenic bacteria like Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa that have been linked to increased inflammation, exacerbation of respiratory symptoms, and disease progression in mouse models (Boutin et al. 2017; Versi et al. 2023). Studies comparing respiratory microbiomes in BALF (bronchoalveolar lavage fluid), and sputum of COPD patients and healthy subjects found increased relative abundance of Moraxella, Streptococcus, Proteobacteria, Veillonella, Eubacterium, and Prevotella sp. in COPD (Ghebre et al. 2018; Haldar et al. 2020; Li et al. 2024).
Another study by Dickson et al. (2017) demonstrated that patients with COPD exhibited a shift in lung microbiome composition compared to healthy controls, with an increase in Proteobacteria and a decrease in Firmicutes. This profile of dysbiosis was associated with increased airway inflammation and exacerbation frequency, highlighting the role of the lung microbiome in disease pathogenesis. Similarly, in cystic fibrosis, dysbiosis characterized by the dominance of opportunistic pathogens such as Pseudomonas aeruginosa, has been linked to progressive lung function decline and poor clinical outcomes (Twomey et al. 2013; Versi et al. 2023). Along with bacterial communities, fungi also have its role to play, as Aspergillus showed the greatest distinction between healthy subjects and individuals with non-CF bronchiectasis, with its prevalence correlating with exacerbation events (Mac Aogain et al. 2018; Li et al. 2024).
Airway diseases: addressing microbial imbalances in respiratory conditions
A study highlighted the association between dysbiosis in the lung microbiome and increased pulmonary inflammation, emphasizing the need to target microbial imbalances for managing respiratory conditions (Segal et al. 2016). Additionally, Dickson et al. (2017) identified specific bacterial topography within the healthy human lower respiratory tract, laying the groundwork for understanding microbial communities in respiratory health and disease. Huang et al. (2015) demonstrated correlations between the airway microbiome composition and disease severity, underlining the potential role of microbial imbalances in exacerbating asthma symptoms. Similarly, in COPD dysbiosis characterized by an overabundance of pathogenic bacteria associated with increased inflammation and exacerbation frequency, suggesting microbial interventions as potential therapeutic targets (Segal et al. 2016). Another study conducted in patients with bronchiectasis demonstrated that P. aeruginosa was independently associated with increased mortality in patients with frequent exacerbations (Araujo et al. 2018). So, managing this specific microbe could be useful in these specific patient groups.
Therapeutic approaches: strategies for enhancing lung microbiome for respiratory well-being
Therapeutic approaches aimed at enhancing the lung microbiome for respiratory well-being are promising avenues for managing airway diseases. Studies have explored diverse strategies to modulate the lung microbiome, including probiotic supplementation, antimicrobial therapy, and microbiota transplantation (Mortaz et al. 2013; Chmiel et al. 2014; Jang et al. 2020; Wu et al. 2021; Du et al. 2022). For example, Yeo et al. (2014) demonstrated the potential of probiotics containing Lactobacillus fermentum CJL-112 strain to prevent influenza virus infection by activating the T-helper cells. In vitro studies have shown that L. rhamnosus NutRes1 possesses the ability to diminish inflammatory mediators produced by smoking-activated human macrophages (Mortaz et al. 2015). Another research conducted on mice models suggests that oral administration of L. paracasei NCC2461 could offer effective protection for female BALB/c mice with asthma (Pellaton et al. 2012; Natalini et al. 2023). Studies also highlighted the importance of targeted antimicrobial therapy in restoring microbial homeostasis and alleviating respiratory symptoms (Pellaton et al. 2012; Natalini et al. 2023).
In a study conducted on adults with idiopathic pulmonary fibrosis, adding co-trimoxazole or doxycycline to standard care did not improve outcomes (Martinez et al. 2021). Antimicrobial therapy also failed to enhance survival or slow lung function deterioration (Chen et al. 2021a, 2021b). In a study on mice, fecal microbiota transplantation coupled with high fiber diet mitigates the progression of emphysema by reducing inflammation and apoptosis. During microbiome analysis it has been seen that bacterial families like Bacteroidaceae and Lachnospiraceae increased after both fecal microbiota transplantation (FMT) and high-fat diet (HFD). These families are known for breaking down fiber into short-chain fatty acids (SCFAs) which may indirectly affect lung health gut-lung axis by modulating systemic inflammation and immune function mediated through SCFA production (Jang et al. 2020). Integrative approaches combining microbiome analysis with host genetics and environmental factors may provide deeper insights into microbial dysbiosis and its impact on respiratory conditions (Cribbs et al. 2016; Singh et al. 2022). With continued advancements in high-throughput sequencing technologies and computational tools, researchers can unravel the complexities of the lung microbiome with unprecedented resolution (Segal et al. 2016; Clarke et al. 2018). Integrating multi-omics approaches and longitudinal studies will provide comprehensive insights into the dynamic nature of the lung microbiome and its role in respiratory diseases (Singh et al. 2022). Ultimately, targeted interventions aimed at modulating the lung microbiome may pave the way for personalized therapeutics and improved outcomes for individuals with respiratory conditions.
Understanding the influence of lung microbiome on gut health (lung-gut axis)
Recent studies have highlighted bidirectional communication pathways between these systems, shedding light on how alterations in one microbiome can affect the other (Madan et al. 2012; Marsland et al. 2015; Dickson et al. 2016; Budden et al. 2017). While the gut and respiratory tract microbiota share similar phylum structures, their dominant bacterial members differ. Actinobacteria, Firmicutes, and Bacteroidetes prevail in the gut, whereas Proteobacteria, Firmicutes, and Bacteroidetes dominate in the lungs (Trivedi and Barve 2020). Recently, Qu et al. (2022) suggested that gut-lung axis plays a significant role in COPD, as the cross talk between the microbial species at both sites are impacting lung health and immunity.
In another study conducted on pediatric cohorts, reduction in the population of A. muciniphila and F. prausnitzii has been associated with an increased risk of allergic asthma in patients. Authors also concluded that possibly F. prausnitzii and A. muciniphila potentially trigger the production of anti-inflammatory cytokine IL-10 while inhibiting pro-inflammatory cytokines like IL-12, indicating their potential to mitigate inflammation via secreted metabolites (Demirci et al. 2019). There is not a lot of clarity if the microbial dysbiosis and altered metabolite production in the gut initiate changes in the immune system and inflammation coupled with disease development in lungs or vice versa (Zhang et al. 2022). Moreover, therapeutic interventions targeting the gut microbiome to improve lung health outcomes have shown promise. Supplementation of Lactobacillus and Streptococcus strains at a particular dose in mice will be beneficial in reducing bacterial counts in the lungs and ultimately helps in maintaining the defense mechanism against S. pneumoniae (Cangemi de Gutierrez et al. 2001; Villena et al. 2006; Fukuda et al. 2011). Fukuda et al. (2011) found that specific microbial metabolites produced by gut commensals, such as short-chain fatty acids (SCFAs), could enhance gut barrier integrity and reduce susceptibility to allergic inflammation. These findings underscore the potential for modulating the gut microbiome to positively influence lung health and overall host immunity.
Skin microbiome
The diverse microbial community on skin is vital for maintaining skin health and homeostasis. Key bacterial species such as Staphylococcus epidermidis and Cutibacterium acnes contribute to skin barrier function, immune regulation, and protection against pathogens (Fourniere et al. 2020). Numerous fungi also inhabit the skin in addition to the bacteria. The fungal species varies depending on the body part involved, for example, Malassezia predominantly harbors on the core body and arms, whereas Aspergillus spp., Cryptococcus spp., Malassezia and Rhodotorula spp., and Epicoccum spp. colonized on the foot (Skowron et al. 2021). Understanding the dynamic interplay within the skin microbiome is essential for elucidating disease mechanisms and developing targeted therapeutic interventions in dermatology. Research on the skin microbiome in various skin diseases reveals dynamic shifts in bacterial composition associated with disease pathogenesis. For instance, in eczema, a decrease in beneficial bacteria such as Staphylococcus epidermidis alongside an increase in potentially pathogenic Staphylococcus aureus has been noted (Skowron et al. 2021). Psoriasis is characterized by alterations in the skin microbiome, including an increase in Streptococcus spp. and reduced bacterial diversity (Celoria et al. 2023). Acne vulgaris is often linked with the colonization of Propionibacterium acnes in hair follicles, and contributes to an inflammatory acne lesion. For the microbiome associated with rosacea, studies suggest potential increases in bacteria like Bacillus oleronius (McLaughlin et al. 2019). These findings underscore the importance of understanding microbial dynamics in skin diseases for developing targeted therapeutic interventions aimed at restoring microbial balance and ameliorating disease symptoms.
Dermatological insights: understanding the microbial impact on skin conditions
Dermatological research has increasingly emphasized the pivotal role of the skin microbiome in the pathogenesis of various skin conditions, providing valuable insights into disease mechanisms and potential therapeutic strategies. Eczema, characterized by compromised skin barrier function and inflammation, demonstrates microbial dysbiosis with a reduction in beneficial Staphylococcus epidermidis and an elevation in pathogenic Staphylococcus aureus (Pessoa et al. 2023; Chaudhary et al. 2023a, 2023b). Similarly, psoriasis, a chronic inflammatory disorder, is associated with alterations in the skin microbiome, including an overabundance of Streptococcus spp. and diminished bacterial diversity (Celoria et al. 2023). Acne vulgaris, one of the most prevalent dermatological conditions globally, involves the colonization of Propionibacterium acnes within pilosebaceous units, contributing to the formation of inflammatory lesions (Vasam et al. 2023). Furthermore, there is emerging evidence implicating the skin microbiome in rosacea, with potential increases in bacteria such as Bacillus oleronius (Daou et al. 2021). Understanding these microbial dynamics not only elucidates disease pathogenesis but also offers avenues for targeted therapeutic interventions aimed at restoring microbial balance and ameliorating disease symptoms, thereby paving the way for personalized dermatological treatments (Byrd et al. 2018; Myles et al. 2018).
Topical treatments: utilizing microbiome for skin health enhancement
Clinical investigations have underscored the efficacy of microbiome-targeted interventions in rebalancing microbial communities, ameliorating inflammation, and fortifying the skin barrier. For instance, formulations enriched with probiotics or prebiotics have demonstrated effectiveness in promoting the growth of beneficial skin bacteria, such as Staphylococcus epidermidis, while concurrently suppressing the proliferation of pathogenic species like Staphylococcus aureus (Di Lodovico et al. 2020). Additionally, postbiotics, including metabolic byproducts of probiotic bacteria, exhibit potent anti-inflammatory and antimicrobial properties, contributing to overall skin health enhancement (Prajapati et al. 2023). Furthermore, emerging therapeutic modalities such as bacteriophage therapy hold promise in selectively targeting and eradicating pathogenic bacteria in the skin microbiome, providing novel avenues for managing skin disorders. In addition, previous studies also reported that Gram negative commensal bacteria Roseomonas mucosa isolated from healthy individuals improved the outcomes of atopic dermatitis in cell culture and mouse models by inhibiting the growth of S. aureus (Myles et al. 2020; Zeldin et al. 2023; Barbian et al. 2023).
Moreover, examples from traditional practices, such as the use of fermented skincare products in East Asian skincare routines, highlight the long-standing recognition of skin microbiome in maintaining skin health and beauty (Jung et al. 2014). These advancements underscore the transformative potential of microbiome-based topical treatments, ushering in a new era in dermatology where personalized, microbiome-centric approaches offer tailored solutions for optimizing skin health and well-being.
Microbiota diversity: the crucial role in skin barrier functionality
Microbiota diversity plays a pivotal role in maintaining the functionality of the skin barrier, serving as a complex ecosystem that interacts with the host immune system and influences various physiological processes. Beneficial bacteria, such as Staphylococcus epidermidis, are key players in promoting skin barrier integrity. S. epidermidis produces antimicrobial peptides, such as bacteriocins, which inhibit the growth of pathogenic microbes and contribute to the overall balance of the skin microbiome (Severn and Horswill 2023). Another example is Cutibacterium acnes (formerly known as Propionibacterium acnes), which produces short-chain fatty acids that help regulate inflammation and lipid production, thereby supporting skin barrier function (Nakatsuji et al. 2017; Almoughrabie et al. 2023).
Conversely, dysbiosis characterized by a decrease in microbial diversity, particularly in beneficial species, can compromise the skin barrier. Reductions in diversity are associated with an overgrowth of pathogenic bacteria like Staphylococcus aureus, which can disrupt the balance of the skin microbiome and contribute to skin barrier dysfunction (Myles et al. 2016). In conditions such as eczema, where the skin barrier is impaired, there is often a decrease in beneficial bacteria like S. epidermidis and an increase in potentially pathogenic species (Kong et al. 2012). Furthermore, alterations in microbial diversity have been observed in various skin disorders, including psoriasis. Studies have shown reduced bacterial diversity in psoriatic skin compared to healthy skin, suggesting a potential role of microbiota diversity in the pathogenesis of the disease (Chen et al. 2020).
Gut connection: exploring how skin microbiome impacts gut health
Perturbations in the gut microbiota can exert systemic effects on the skin microbiome, impacting skin health and contributing to the pathogenesis of various dermatological conditions. For instance, dysbiosis in the gut microbiota can lead to systemic inflammation and immune dysregulation, which may manifest as skin disorders such as eczema, psoriasis, and acne (Belkaid and Segre 2014; Salem et al. 2018; Pessoa et al. 2023). Conversely, disruptions in the skin microbiome, such as alterations in microbial diversity and composition, can influence gut health through systemic inflammation and the modulation of immune responses (Mahmud et al. 2022). Prominent bacterial species implicated in this crosstalk include Staphylococcus epidermidis and Cutibacterium acnes, which play key roles in maintaining skin barrier function and immune homeostasis (Fourniere et al. 2020; Zhang et al. 2024).
The promotion of gut health through dietary interventions, probiotics, and prebiotics has emerged as a promising therapeutic approach for alleviating skin diseases (Ji et al. 2023).
Probiotics, such as Lactobacillus and Bifidobacterium species, have been shown to enhance gut barrier function, modulate immune responses, and alleviate inflammatory skin conditions like atopic dermatitis (Navarro-Lopez et al. 2019; Gao et al. 2023; Xie et al. 2023). Prebiotics, including dietary fibers and oligosaccharides, promote the growth of beneficial gut bacteria, leading to improvements in gut microbiota composition and function (O’Callaghan and van Sinderen 2016; Fu et al. 2022; Lee et al. 2023). These interventions can mitigate systemic inflammation and restore microbial balance, thereby ameliorating skin diseases and promoting skin barrier integrity (Jung et al. 2014; Tokarek et al. 2021; Gao et al. 2023). Furthermore, emerging research highlights the role of the gut-skin axis in the pathogenesis of autoimmune skin disorders such as psoriasis and vitiligo (Navarro-Lopez et al. 2019; Thye et al. 2022). Dysregulation of the immune system in the gut can trigger inflammatory cascades that contribute to skin inflammation and tissue damage (Yoo et al. 2020; Maciel-Fiuza et al. 2023). Modulation of the gut microbiota through interventions such as fecal microbiota transplantation (FMT) and dietary modifications has shown promise in attenuating autoimmune skin diseases by restoring immune homeostasis and dampening inflammatory responses (Navarro-Lopez et al. 2019; Kim et al. 2021). By elucidating the mechanisms underlying the gut-skin axis and harnessing the therapeutic potential of microbiome-targeted interventions, clinicians can optimize patient outcomes and improve the quality of life for individuals with dermatological conditions (Baud et al. 2023).
Vaginal microbiome
The vaginal microbiome is a complex and dynamic microecosystem that undergoes changes during the menstrual cycle as well as in the entire life of the woman. The vaginal mucosa comprised stratified squamous nonkeratinized epithelium covered by cervicovaginal secretion and it procures glucose, oxygen, and other nutrients from underlying mucosal tissues via the process of diffusion due to limited blood supply (Chen et al. 2021a, 2021b). The vagina harbors a complex consortium of microbial communities that lives in a symbiotic relationship with the host (Chaudhary et al. 2023a, 2023b). The vaginal microecosystem harbors a billion microbes in a healthy vagina and among all, Lactobacillus is a dominant microbe that is known to produce numerous antimicrobial compounds. A survey by Chen et al. (2021a, b) suggested that the vagina contains 1010–1011 bacteria in women of reproductive age.
Women’s health: maintaining microbial balance in vaginal ecology
Maintaining microbial balance in vaginal ecology is crucial for health of women, as it directly influences various aspects of reproductive and overall well-being (Lehtoranta et al. 2022). The vaginal microbiota, predominantly composed of bacteria, plays a pivotal role in protecting against infections, maintaining pH balance, and supporting reproductive health (Baud et al. 2023). The healthy abundance of Lactobacillus species produces lactic acid to create an acidic environment hostile to pathogenic microorganisms. This acidic pH helps prevent the overgrowth of harmful bacteria, fungi, and other pathogens, reducing the risk of conditions such as bacterial vaginosis, yeast infections, and sexually transmitted infections (STIs) (Lin et al. 2021). Factors such as hormonal fluctuations, sexual activity, hygiene practices, diet, and use of antibiotics can disrupt the delicate balance of vaginal microbiota, leading to dysbiosis or an imbalance in microbial composition (Tuniyazi and Zhang 2023). Dysbiosis can result in symptoms like abnormal vaginal discharge, itching, burning, and increased susceptibility to infections.
Maintaining microbial balance in the vaginal ecosystem involves adopting practices that support the growth of beneficial bacteria while minimizing factors that promote the proliferation of harmful pathogens. One key aspect is practicing good hygiene, including gentle cleansing of the external genital area with mild, unscented soap and water. It is important to avoid douching and using scented products in the genital area, as these can disrupt the vaginal microbiota. Additionally, maintaining a healthy diet rich in fruits, vegetables, and probiotic-rich foods can support vaginal health by promoting the growth of beneficial bacteria. Probiotics, either through dietary sources or supplements, can help replenish and maintain the population of Lactobacillus species in the vagina that aides in the prevention of infections and maintaining a balanced microbial ecosystem (Chen et al. 2021a, 2021b; Wang et al. 2021a, 2021b).
Screening for STIs, bacterial vaginosis, and yeast infections allows for timely intervention and treatment, minimizing the risk of complications and promoting overall vaginal health (Chen et al. 2021a, 2021b). Healthcare providers may recommend targeted treatments such as antimicrobial medications or probiotic supplements specifically formulated for vaginal health to women experiencing recurrent vaginal infections or dysbiosis. These interventions aim to restore microbial balance and alleviate symptoms associated with vaginal dysbiosis (Campisciano et al. 2021).
It is also important for women to be aware of the impact of certain lifestyle choices on vaginal health. Smoking, excessive alcohol consumption, stress, and unprotected sexual activity with multiple partners can disrupt the vaginal microbiota and increase the risk of infections (Lewis et al. 2017; Froehle et al. 2021; Turpin et al. 2021). Pregnant women should receive regular prenatal care, including screenings for vaginal infections, to ensure optimal vaginal health for themselves and their babies (Baud et al. 2023).
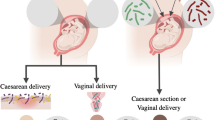
Reproductive health: understanding microbiota’s role in pregnancy and birth
Understanding the role of microbiota in pregnancy and birth is crucial for promoting reproductive health and ensuring positive maternal and neonatal outcomes. The human microbiota, including the vaginal, gut, and placental microbiota, undergoes significant changes throughout pregnancy, influencing various aspects of maternal health, fetal development, and birth outcomes. The vaginal microbiota plays a critical role in pregnancy by providing protection against infections and maintaining a healthy environment for fetal development (Ravel et al. 2011). During pregnancy, the composition of the vaginal microbiota may shift, with an increase in Lactobacillus species associated with a lower risk of adverse pregnancy outcomes such as preterm birth (Aagaard et al. 2014). The gut microbiota also plays a crucial role in pregnancy by influencing maternal immune function, nutrient metabolism, and inflammation (Collado et al. 2008). Dysbiosis of the gut microbiota during pregnancy has been associated with gestational diabetes, obesity, and other metabolic disorders (Li et al. 2021).
Furthermore, studies have highlighted the presence of a placental microbiota, challenging the traditional notion of a sterile intrauterine environment (Aagaard et al. 2014). The placental microbiota may play a role in fetal development and immune programming, although further research is needed to fully understand its impact on pregnancy outcomes. Understanding the role of microbiota in pregnancy and birth has significant implications for maternal and neonatal healthcare (Yao et al. 2021). Strategies aimed at promoting healthy microbiota during pregnancy, such as probiotic supplementation, dietary interventions, and targeted antibiotic use, may help reduce the risk of complications and improve birth outcomes (Obuchowska et al. (n.d.); Obuchowska et al. 2022). Additionally, advances in microbiome research have led to the development of novel diagnostic and therapeutic approaches for managing pregnancy-related conditions. Biomarkers derived from maternal microbiota profiles may offer valuable insights into the risk of preterm birth, preeclampsia, and other pregnancy complications, enabling early intervention and personalized care (Kumar et al. 2021).
Probiotic interventions: strategies for harmonizing the vaginal microbiome
Probiotic interventions offer a promising strategy for harmonizing the vaginal microbiome, promoting women’s health, and preventing or treating various vaginal dysbiosis-related conditions. Probiotics are live microorganisms that when administered in adequate amounts, confer health benefits to the host by restoring microbial balance and enhancing immune function. Lactobacillus species, particularly Lactobacillus crispatus, Lactobacillus jensenii, Lactobacillus gasseri, and Lactobacillus iners, are commonly found in healthy vaginal microbiota that plays a crucial role in maintaining vaginal health (Ravel et al. (n.d.)). Probiotic supplements containing these Lactobacillus strains (L. crispatus DSM32720, L. rhamnosus HN001, L. crispatus LMG S-29995, L. acidophilus GLA-14 and L. crispatus DSM32717) have been shown to effectively restore and maintain a balanced vaginal microbiome by increasing the abundance of beneficial bacteria and reducing the risk of vaginal infections (Liu et al. 2023). Several clinical studies have demonstrated the efficacy of probiotic interventions in preventing recurrent bacterial vaginosis (BV) and yeast infections (Reid et al. 2001; Falagas et al. 2006). Probiotic formulations containing Lactobacillus strains have been shown to reduce the recurrence rate of BV and restore vaginal pH to a healthy acidic range, thereby preventing pathogenic bacteria from colonizing the vagina (Mei and Li 2022).
In addition to preventing vaginal infections, probiotics may also have therapeutic potential in managing other vaginal dysbiosis-related conditions, such as vulvovaginal candidiasis (VVC) and aerobic vaginitis (AV) (Liu et al. 2023). Probiotic strains with antimicrobial properties can compete with and inhibit the growth of pathogenic microorganisms, restoring microbial balance and alleviating symptoms associated with these conditions (Plaza-Diaz et al. 2018). Furthermore, probiotic interventions have been investigated as adjunctive therapy for the treatment of sexually transmitted infections (STIs) such as bacterial vaginosis (BV) and human papillomavirus (HPV) infection (Liu et al. 2023). By modulating the vaginal microbiome and enhancing immune function, probiotics may help reduce the risk of STI acquisition and transmission, although further research is needed to confirm their efficacy in this context (Santos et al. 2023).
When considering probiotic interventions for vaginal health, it is essential to select probiotic strains with proven efficacy and safety profiles Lactobacillus (Lacticaseibacillus rhamnosus MG4288, Lactiplantibacillus plantarum MG989, Lacticaseibacillus paracasei MG4272, Ligilactobacillus salivarius MG242, and Limosilactobacillus fermentum MG901 species are the most used probiotics for vaginal health (Park et al. 2023).
Gut connection: exploring the influence of vaginal microbiome on gut health
The relationship between the vaginal microbiome and gut health is an emerging area of research that highlights the interconnectedness of these two microbial ecosystems and their impact on overall health (Mestrovic et al. 2020). Studies have shown that alterations in the vaginal microbiome can influence gut health and vice versa. For example, changes in vaginal microbial composition, such as a decrease in Lactobacillus species and an increase in pathogenic bacteria, have been associated with gastrointestinal (GI) disorders such as irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD) (Yeoh et al. 2021; Bar et al. 2023). Conversely, disturbances in composition of gut microbiota, often induced by factors like diet, stress, antibiotics, and infections, can affect vaginal health by disrupting hormonal balance, immune function, and microbial diversity. Dysbiosis of the gut microbiota has been linked to an increased risk of vaginal infections, including BV and VVC (Tuddenham et al. 2019; Han et al. 2021). Furthermore, emerging evidence suggests that the transmission of microbial communities between the gut and vagina may occur through various routes, including the bloodstream, mucosal surfaces, and shared anatomical pathways (Takada et al. 2023) .
Probiotic supplementation is one promising approach for modulating both vaginal and gut microbiota composition and promoting overall health. Lactobacillus crispatus and Lactobacillus rhamnosus BMX 54 are the commonly used strains in probiotic formulations for vaginal health and confer benefits to gut health by enhancing immune function, reducing inflammation, and inhibiting the growth of pathogenic bacteria (Recine et al. 2016; Kyser et al. 2023). Similarly, probiotics containing beneficial bacteria such as Bifidobacterium and Lactobacillus species have demonstrated efficacy in restoring vaginal microbial balance and preventing or treating vaginal infections. By promoting a balanced microbiome in both the gut and vagina, probiotic interventions may contribute to improved overall health outcomes. Understanding the influence of the vaginal microbiome on gut health and vice versa opens new avenues for research and therapeutic interventions aimed at promoting holistic approaches to health and wellness.
Gut microbiome
The gut microbiome housing a diverse spectrum of microorganisms thriving within the gastrointestinal tract forms a dynamic ecosystem vital for human health (Qin et al. 2012; Thursby and Juge 2017). Influenced by a plethora of factors such as host genetics, dietary habits, age, antibiotic administration, and environmental exposures, the equilibrium of the gut microbiome is intricately balanced and easily perturbed (Lynch and Pedersen 2016). Such disruptions, referred to as dysbiosis, have been implicated in the onset of numerous diseases, spanning inflammatory bowel disorders, metabolic irregularities, autoimmune afflictions, and even neurological conditions (Lynch and Pedersen 2016; Marchesi et al. 2016). The connection between different body regions and diseases stemming from microbial imbalance is explored, identifying particular microbial groups showing excess and deficiency. Furthermore, it discusses the use of probiotic treatments to address these specific conditions (Table 1).
The impact of the gut microbiome on health and disease: insights into therapeutic strategies
Beneficial bacteria such as Bifidobacterium and Lactobacillus contribute to nutrient metabolism, synthesis of vitamins, and maintenance of intestinal barrier integrity, thus promoting digestive function (Round and Mazmanian 2009; Hill et al. 2014). Moreover, the gut microbiome interacts with the immune system, modulating immune responses and protecting against pathogens, which is essential for maintaining gastrointestinal health (Belkaid and Hand 2014). Dysbiosis of the gut microbiome, characterized by alterations in microbial composition and diversity, is associated with various digestive disorders including irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), and colorectal cancer (Menees and Chey 2018; Kim and Lee 2021; Shan et al. 2022).
The microbiome of gut influence extends beyond digestion, impacting various physiological processes crucial for health maintenance. These include the regulation of metabolism, neurotransmitter production, and even immune system development (Sommer and Backhed 2013; Dicks 2022). Dysregulation of the gut microbiome has been linked to metabolic disorders like obesity and type 2 diabetes, as well as neurodevelopmental conditions such as autism spectrum disorder and depression ((Tilg and Kaser 2011; Mayer et al. 2014; Liu et al. 2020). Furthermore, disruptions in gut microbial composition have been associated with systemic inflammation, contributing to the pathogenesis of chronic diseases like cardiovascular disease and rheumatoid arthritis (Scherer et al. 2020). The emerging field of microbiome research offers insights into novel therapeutic approaches, including precision medicine strategies targeting the gut microbiota to prevent and treat a diverse range of health conditions (Lloyd-Price et al. 2016; Marchesi et al. 2016).
Gut-brain axis: investigating the connection between gut microbiome and mood
Emerging research has highlighted the profound impact of gut microbiota on mood regulation and emotional well-being. Beneficial gut bacteria, including species like Bifidobacterium and Lactobacillus, are responsible for synthesizing neurotransmitters such as serotonin and gamma-aminobutyric acid (GABA), which are pivotal in regulating mood (Cryan and Dinan 2012; Foster and McVey Neufeld 2013). In a recent study, the efficacy of a mixture containing Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 was examined, revealing favorable psychological outcomes alongside (modulating the gut microbiota, increase in short chain fatty acids and gamma-aminobutyric acid, decrease in pro-inflammatory cytokines, and increase in anti-inflammatory cytokines) decreased serum cortisol levels in human participants (De Oliveira et al. 2023). Moreover, gut microbes influence the production of neuroactive compounds, including short-chain fatty acids (SCFAs) have the ability to influence brain function and behavior (Cryan and Dinan 2012). Dysbiosis of the gut microbiome has been implicated in the pathogenesis of mood disorders such as depression and anxiety, with alterations in microbial composition and diversity observed in affected individuals particularly with decrease in microbial diversity and richness (Jiang et al. 2015; Kelly et al. 2016). Patients of depressive order have higher abundance of Enterobacteriaceae and Alistipes and decreased level of fecal bacterium abundance.
The bidirectional communication along the gut-brain axis involves intricate signaling pathways, including neural, endocrine, and immune mechanisms, that influence mood and cognitive function (Mayer and Tillisch 2011; Foster and McVey Neufeld 2013). Perturbations in the gut microbiome, induced by factors such as stress, diet, and antibiotic use, can disrupt this communication, contributing to alterations in mood and behavior (Foster and McVey Neufeld 2013; Kelly et al. 2016). Additionally, inflammation triggered by dysbiosis of the gut microbiome has been implicated in the pathophysiology of mood disorders, highlighting the role of immune activation in modulating brain function and mental health (Rook et al. 2014; Dinan and Cryan 2017). The integration of microbiome-targeted interventions, including probiotics, dietary modifications, and fecal microbiota transplantation (FMT), holds promise for restoring microbial balance and alleviating symptoms of mood disorders by targeting the gut-brain axis (Slyepchenko et al. 2017; Wallace and Milev 2017) personalized approaches to mental health care.
Dietary strategies: promoting gut microbiome diversity for overall health benefits
Promoting gut health through dietary interventions and supplements is essential for maintaining microbial balance and supporting overall well-being. A diet rich in fiber, fruits, vegetables, and fermented foods serves as a cornerstone for gut health by providing prebiotic fibers that nourish beneficial gut bacteria (Fu et al. 2022; Bedu-Ferrari et al. 2022). Prebiotics, such as inulin, oligofructose, and resistant starch, selectively stimulate the growth of beneficial microbes like Bifidobacteria and Lactobacilli, thereby enhancing gut microbial diversity and function (Holscher 2017; Baxter et al. 2019). Probiotic-rich foods like yogurt, kefir, and kimchi contain strains of Lactobacillus and Bifidobacterium that support gut health and may alleviate gastrointestinal symptoms (Hill et al. 2014; Leeuwendaal et al. 2022).
Furthermore, dietary supplements such as prebiotics and probiotics offer convenient ways to enhance gut health (Holscher 2017; Fu et al. 2022). Moreover, synbiotic formulations, which combine prebiotics and probiotics, synergistically promote gut health by providing both substrate and live bacteria for microbial fermentation and growth (Gibson and Roberfroid 1995).
In addition to dietary interventions and supplements, lifestyle factors such as stress management, regular exercise, and adequate sleep also play crucial roles in promoting gut health. Chronic stress and sleep disturbances can disrupt gut microbial composition and function, leading to gastrointestinal symptoms and systemic inflammation (Kelly et al. 2016). Age is also an important factor which can change microbiome; elderly individuals exhibited altered abundance of 21 microbial species, indicating age-related shifts in gut and oral microbiomes in Singaporean population (Chen et al. 2022). Overall, promoting gut health through dietary interventions, supplements, and lifestyle modifications is essential for maintaining microbial balance, supporting digestive function, and enhancing overall health and well-being.
Challenges in adopting microbiome modulation interventions
Implementing interventions to modulate the microbiome for improved systemic health and reduced inflammation faces several hurdles. Firstly, the vast complexity and diversity of the human microbiome pose challenges in developing universally applicable interventions that effectively target specific microbial imbalances. Additionally, the lack of standardized protocols for dosage, duration, and administration methods complicates consistent implementation in clinical practice. Furthermore, limited understanding of microbiome-host interactions and potential adverse effects raises safety concerns among healthcare providers. Cost and accessibility issues also restrict widespread adoption, as some interventions may be costly or unavailable to certain populations. Moreover, regulatory hurdles prolong the development and approval of novel interventions, further limiting their availability for clinical use. Addressing these challenges requires ongoing research efforts to enhance our understanding of the microbiome and develop safe, effective, and accessible interventions, alongside efforts to standardize protocols and streamline regulatory processes.
Concluding remarks
In conclusion, this review has illuminated the individual role of site-specific microbiomes in health and diseases and intricate interplay between various microbiomes throughout the body, ranging from the oral cavity and lungs to the vagina, skin, and the central hub of the gut. Understanding these microbial communities and their connections is pivotal in unraveling the complexities of human health and disease. Through extensive research, it has become increasingly evident that the gut microbiota plays a crucial role in modulating the health of other microbiome sites and vice versa. Bidirectional communication between the gut and distant microbiomes influences physiological processes, immune responses, and susceptibility to various disorders. Moreover, the emerging field of microbiome-based therapies holds tremendous promise in revolutionizing healthcare. Harnessing the therapeutic potential of gut microbiota in the form of probiotics and fecal transplantation therapies to modulate distant microbiomes offers a novel approach for treating a myriad of diseases. From oral infections to respiratory disorders, vaginal dysbiosis, dermatological conditions, and beyond, interventions targeting the gut microbiome could pave the way for personalized, effective treatments. As we continue to delve deeper into the complexities of the microbiome and its systemic effects, interdisciplinary collaboration and innovative research methodologies will be essential. By unraveling the intricate connections between different microbiomes and leveraging the therapeutic potential of the gut microbiota, we can aspire to develop targeted interventions that promote health and combat disease across multiple body sites. This journey toward microbiome-centric healthcare holds immense promise for improving the well-being of individuals worldwide.
References
Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J (2014) The placenta harbors a unique microbiome. Sci Transl Med 6:237ra265
Ahmed A, Dachang W, Lei Z, Jianjun L, Juanjuan Q, Yi X (2014) Effect of Lactobacillus species on Streptococcus mutans biofilm formation. Pak J Pharm Sci 27:1523–1528
Allaker RP, Stephen AS (2017) Use of probiotics and oral health. Curr Oral Health Rep 4:309–318
Almoughrabie S, Cau L, Cavagnero K, O’Neill AM, Li F, Roso-Mares A, Mainzer C, Closs B, Kolar MJ, Williams KJ (2023) Commensal Cutibacterium acnes induce epidermal lipid synthesis important for skin barrier function. Sci Adv 9:eadg6262
Amabebe E, Anumba DOC (2020) Female gut and genital tract microbiota-induced crosstalk and differential effects of short-chain fatty acids on immune sequelae. Front Immunol 11:2184
Araujo D, Shteinberg M, Aliberti S, Goeminne PC, Hill AT, Fardon TC, Obradovic D, Stone G, Trautmann M, Davis A (2018) The independent contribution of Pseudomonas aeruginosa infection to long-term clinical outcomes in bronchiectasis. Eur Respir J 51
Atarashi K, Suda W, Luo C, Kawaguchi T, Motoo I, Narushima S, Kiguchi Y, Yasuma K, Watanabe E, Tanoue T (2017) Ectopic colonization of oral bacteria in the intestine drives T(H)1 cell induction and inflammation. Science 358:359–365
Bar O, Sudhof LS, Yockey LJ, Bergerat A, Moriel N, Andrews E, Ananthakrishnan AN, Xavier RJ, Yassour M, Mitchell CM (2023) Comparison of vaginal microbiota between women with inflammatory bowel disease and healthy controls. PLoS One 18:e0284709
Barbian K, Bruno D, Sykora L, Ricklefs S, Chaudhary PP, Beare PA, Myles IA, Martens CM (2023) De novo assembly of Roseomonas mucosa isolated from patients with atopic dermatitis. Microbiol Resour Announc 12:e0052123
Baud A, Hillion KH, Plainvert C, Tessier V, Tazi A, Mandelbrot L, Poyart C, Kennedy SP (2023) Microbial diversity in the vaginal microbiota and its link to pregnancy outcomes. Sci Rep 13:9061
Baxter MFA, Latorre JD, Dridi S, Merino-Guzman R, Hernandez-Velasco X, Hargis BM, Tellez-Isaias G (2019) Identification of serum biomarkers for intestinal integrity in a broiler chicken malabsorption model. Front Vet Sci 6:144
Bedu-Ferrari C, Biscarrat P, Langella P, Cherbuy C (2022) Prebiotics and the human gut microbiota: from breakdown mechanisms to the impact on metabolic health. Nutrients 14
Belkaid Y, Hand TW (2014) Role of the microbiota in immunity and inflammation. Cell 157:121–141
Belkaid Y, Segre JA (2014) Dialogue between skin microbiota and immunity. Science 346:954–959
Borrell Garcia C, Ribelles Llop M, Garcia Esparza MA, Flichy-Fernandez AJ, Marques Martinez L, Izquierdo Fort R (2021) The use of Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 on oral health indexes in a school population: a pilot randomized clinical trial. Int J Immunopathol Pharmacol 35:20587384211031107
Boutin S, Graeber SY, Stahl M, Dittrich AS, Mall MA, Dalpke AH (2017) Chronic but not intermittent infection with Pseudomonas aeruginosa is associated with global changes of the lung microbiome in cystic fibrosis. Eur Respir J 50
Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, Hansbro PM (2017) Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol 15:55–63
Byrd AL, Belkaid Y, Segre JA (2018) The human skin microbiome. Nat Rev Microbiol 16:143–155
Campbell CD, Gleeson M, Sulaiman I (2023) The role of the respiratory microbiome in asthma. Front Allergy 4:1120999
Campisciano G, Zanotta N, Petix V, Giangreco M, Ricci G, Maso G, Comar M, De Seta F (2021) Vaginal dysbiosis and partial bacterial vaginosis: the interpretation of the “grey zones” of clinical practice. Diagnostics (Basel) 11
Cangemi de Gutierrez R, Santos V, Nader-Macias ME (2001) Protective effect of intranasally inoculated Lactobacillus fermentum against Streptococcus pneumoniae challenge on the mouse respiratory tract. FEMS Immunol Med Microbiol 31:187–195
Carabotti M, Scirocco A, Maselli MA, Severi C (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol 28:203–209
Celoria V, Rosset F, Pala V, Dapavo P, Ribero S, Quaglino P, Mastorino L (2023) The skin microbiome and its role in psoriasis: a review. Psoriasis (Auckl) 13:71–78
Chaudhary PP, Myles IA, Zeldin J, Dabdoub S, Deopujari V, Baveja R, Baker R, Bengtson S, Sutton A, Levy S, Hourigan SK (2023a) Shotgun metagenomic sequencing on skin microbiome indicates dysbiosis exists prior to the onset of atopic dermatitis. Allergy 78:2724–2731
Chaudhary PP, O’Laughlin B, Kumar PS, Dabdoub SM, Levy S, Myles IA, Hourigan SK (2023b) Vaginal delivery provides skin colonization resistance from environmental microbes in the NICU. Clin Transl Med 13:e1506
Chen CY, Chen CH, Wang CY, Lai CC, Chao CM, Wei YF (2021a) The effect of additional antimicrobial therapy on the outcomes of patients with idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Respir Res 22:243
Chen L, Li J, Zhu W, Kuang Y, Liu T, Zhang W, Chen X, Peng C (2020) Skin and gut microbiome in psoriasis: gaining insight into the pathophysiology of it and finding novel therapeutic strategies. Front Microbiol 11:589726
Chen L, Zheng T, Yang Y, Chaudhary PP, Teh JPY, Cheon BK, Moses D, Schuster SC, Schlundt J, Li J, Conway PL (2022) Integrative multiomics analysis reveals host-microbe-metabolite interplays associated with the aging process in Singaporeans. Gut Microbes 14:2070392
Chen X, Lu Y, Chen T, Li R (2021b) The female vaginal microbiome in health and bacterial vaginosis. Front Cell Infect Microbiol 11:631972
Chmiel JF, Aksamit TR, Chotirmall SH, Dasenbrook EC, Elborn JS, LiPuma JJ, Ranganathan SC, Waters VJ, Ratjen FA (2014) Antibiotic management of lung infections in cystic fibrosis. I. The microbiome, methicillin-resistant Staphylococcus aureus, gram-negative bacteria, and multiple infections. Ann Am Thorac Soc 11:1120–1129
Clarke EL, Lauder AP, Hofstaedter CE, Hwang Y, Fitzgerald AS, Imai I, Biernat W, Rekawiecki B, Majewska H, Dubaniewicz A (2018) Microbial lineages in sarcoidosis. A metagenomic analysis tailored for low-microbial content samples. Am J Respir Crit Care Med 197:225–234
Collado MC, Isolauri E, Laitinen K, Salminen S (2008) Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr 88:894–899
Cookson W, Cox MJ, Moffatt MF (2018) New opportunities for managing acute and chronic lung infections. Nat Rev Microbiol 16:111–120
Cribbs SK, Uppal K, Li S, Jones DP, Huang L, Tipton L, Fitch A, Greenblatt RM, Kingsley L, Guidot DM (2016) Correlation of the lung microbiota with metabolic profiles in bronchoalveolar lavage fluid in HIV infection. Microbiome 4:3
Cryan JF, Dinan TG (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci 13:701–712
Daou H, Paradiso M, Hennessy K, Seminario-Vidal L (2021) Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb) 11:1–12
Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8:481–490
De Oliveira FL, Salgaco MK, de Oliveira MT, Mesa V, Sartoratto A, Peregrino AM, Ramos WS, Sivieri K (2023) Exploring the potential of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 as promising psychobiotics Using SHIME. Nutrients 15
Dekaboruah E, Suryavanshi MV, Chettri D, Verma AK (2020) Human microbiome: an academic update on human body site specific surveillance and its possible role. Arch Microbiol 202:2147–2167
Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, Cokugras H, Kocazeybek BS (2019) Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) 47:365–371
Dewhirst FE, Chen T, Izard J, Paster BJ, Tanner AC, Yu WH, Lakshmanan A, Wade WG (2010) The human oral microbiome. J Bacteriol 192:5002–5017
Di Lodovico S, Gasparri F, Di Campli E, Di Fermo P, D’Ercole S, Cellini L, Di Giulio M (2020) Prebiotic combinations effects on the colonization of Staphylococcal skin strains. Microorganisms 9
Dicks LMT (2022) Gut Bacteria and Neurotransmitters. Microorganisms 10
Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, Curtis JL (2017) Bacterial topography of the healthy human lower respiratory tract. mBio 8
Dickson RP, Erb-Downward JR, Huffnagle GB (2013) The role of the bacterial microbiome in lung disease. Expert Rev Respir Med 7:245–257
Dickson RP, Singer BH, Newstead MW, Falkowski NR, Erb-Downward JR, Standiford TJ, Huffnagle GB (2016) Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat Microbiol 1:16113
Dinan TG, Cryan JF (2017) The microbiome-gut-brain axis in health and disease. Gastroenterol Clin North Am 46:77–89
Du T, Lei A, Zhang N, Zhu C (2022) The beneficial role of probiotic Lactobacillus in respiratory diseases. Front Immunol 13:908010
Elzayat H, Mesto G, Al-Marzooq F (2023) Unraveling the impact of gut and oral microbiome on gut health in inflammatory bowel diseases. Nutrients 15
Enaud R, Prevel R, Ciarlo E, Beaufils F, Wieers G, Guery B, Delhaes L (2020) The gut-lung axis in health and respiratory diseases: a place for inter-organ and inter-kingdom crosstalks. Front Cell Infect Microbiol 10:9
Erb-Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA, Young VB, Toews GB, Curtis JL, Sundaram B (2011) Analysis of the lung microbiome in the “healthy” smoker and in COPD. PLoS One 6:e16384
Falagas ME, Betsi GI, Athanasiou S (2006) Probiotics for prevention of recurrent vulvovaginal candidiasis: a review. J Antimicrob Chemother 58:266–272
Foster JA, McVey Neufeld KA (2013) Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312
Fourniere M, Latire T, Souak D, Feuilloley MGJ, Bedoux G (2020) Staphylococcus epidermidis and Cutibacterium acnes: two major sentinels of skin microbiota and the influence of cosmetics. Microorganisms 8
Froehle L, Ghanem KG, Page K, Hutton HE, Chander G, Hamill MM, Gilliams E, Tuddenham S (2021) Bacterial vaginosis and alcohol consumption: a cross-sectional retrospective study in Baltimore, Maryland. Sex Transm Dis 48:986–990
Fu J, Zheng Y, Gao Y, Xu W (2022) Dietary fiber intake and gut microbiota in human health. Microorganisms 10
Fukuda S, Toh H, Hase K, Oshima K, Nakanishi Y, Yoshimura K, Tobe T, Clarke JM, Topping DL, Suzuki T (2011) Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469:543–547
Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F (2018) Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 9:488–500
Gao T, Wang X, Li Y, Ren F (2023) The role of probiotics in skin health and related gut-skin axis: a review. Nutrients 15
Garcia-Nunez M, Millares L, Pomares X, Ferrari R, Perez-Brocal V, Gallego M, Espasa M, Moya A, Monso E (2014) Severity-related changes of bronchial microbiome in chronic obstructive pulmonary disease. J Clin Microbiol 52:4217–4223
Genco RJ, Borgnakke WS (2013) Risk factors for periodontal disease. Periodontol 2000 62:59–94
Ghebre MA, Pang PH, Diver S, Desai D, Bafadhel M, Haldar K, Kebadze T, Cohen S, Newbold P, Rapley L (2018) Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol 141:2027–2036.e2012
Gibson GR, Roberfroid MB (1995) Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr 125:1401–1412
Hajishengallis G (2015) Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 15:30–44
Haldar K, George L, Wang Z, Mistry V, Ramsheh MY, Free RC, John C, Reeve NF, Miller BE, Tal-Singer R (2020) The sputum microbiome is distinct between COPD and health, independent of smoking history. Respir Res 21:183
Han Y, Liu Z, Chen T (2021) Role of vaginal microbiota dysbiosis in gynecological diseases and the potential interventions. Front Microbiol 12:643422
Haukioja A (2010) Probiotics and oral health. Eur J Dent 4:348–355
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11:506–514
Holscher HD (2017) Dietary fiber and prebiotics and the gastrointestinal microbiota. Gut Microbes 8:172–184
Hou K, Wu ZX, Chen XY, Wang JQ, Zhang D, Xiao C, Zhu D, Koya JB, Wei L, Li J, Chen ZS (2022) Microbiota in health and diseases. Signal Transduct Target Ther 7:135
Huang YJ et al (2015) The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 136(4):874–884
Huffnagle GB, Dickson RP, Lukacs NW (2017) The respiratory tract microbiome and lung inflammation: a two-way street. Mucosal Immunol 10:299–306
Jang YO, Lee SH, Choi JJ, Kim DH, Choi JM, Kang MJ, Oh YM, Park YJ, Shin Y, Lee SW (2020) Fecal microbial transplantation and a high fiber diet attenuates emphysema development by suppressing inflammation and apoptosis. Exp Mol Med 52:1128–1139
Ji J, Jin W, Liu SJ, Jiao Z, Li X (2023) Probiotics, prebiotics, and postbiotics in health and disease. MedComm 4:e420
Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194
Jung JY, Kwon HH, Hong JS, Yoon JY, Park MS, Jang MY, Suh DH (2014, 94) Effect of dietary supplementation with omega-3 fatty acid and gamma-linolenic acid on acne vulgaris: a randomised, double-blind, controlled trial. Acta Derm Venereol:521–525
Kato T, Yamazaki K, Nakajima M, Date Y, Kikuchi J, Hase K, Ohno H, Yamazaki K (2018) Oral administration of Porphyromonas gingivalis alters the gut microbiome and serum metabolome. mSphere 3
Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G (2016) Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118
Kho ZY, Lal SK (2018) The Human Gut Microbiome - A Potential Controller of Wellness and Disease. Front Microbiol 9:1835
Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, Tonetti MS, Wade WG, Zaura E (2016) The oral microbiome - an update for oral healthcare professionals. Br Dent J 221:657–666
Kim J, Lee HK (2021) Potential role of the gut microbiome in colorectal cancer progression. Front Immunol 12:807648
Kim JH, Kim K, Kim W (2021) Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy. Exp Mol Med 53:907–916
Kong HH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, Nomicos E, Polley EC, Komarow HD, Program NCS (2012) Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res 22:850–859
Kumar M, Saadaoui M, Elhag DA, Murugesan S, Al Abduljabbar S, Fagier Y, Ortashi O, Abdullahi H, Ibrahim I, Alberry M (2021) Omouma: a prospective mother and child cohort aiming to identify early biomarkers of pregnancy complications in women living in Qatar. BMC Pregnancy Childbirth 21:570
Kyser AJ, Masigol M, Mahmoud MY, Ryan M, Lewis WG, Lewis AL, Frieboes HB, Steinbach-Rankins JM (2023) Fabrication and characterization of bioprints with Lactobacillus crispatus for vaginal application. J Control Release 357:545–560
Lamont RJ, Koo H, Hajishengallis G (2018) The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 16:745–759
Lee DH, Seong H, Chang D, Gupta VK, Kim J, Cheon S, Kim G, Sung J, Han NS (2023) Evaluating the prebiotic effect of oligosaccharides on gut microbiome wellness using in vitro fecal fermentation. NPJ Sci Food 7:18
Leeuwendaal NK, Stanton C, O’Toole PW, Beresford TP (2022) Fermented foods, health and the gut microbiome. Nutrients 14
Lehtoranta L, Ala-Jaakkola R, Laitila A, Maukonen J (2022) Healthy vaginal microbiota and influence of probiotics across the female life span. Front Microbiol 13:819958
Lewis FMT, Bernstein KT, Aral SO (2017) Vaginal microbiome and its relationship to behavior, sexual health, and sexually transmitted diseases. Obstet Gynecol 129:643–654
Li R, Li J, Zhou X (2024) Lung microbiome: new insights into the pathogenesis of respiratory diseases. Signal Transduct Target Ther 9:19
Li X, Liu Y, Yang X, Li C, Song Z (2022a) The oral microbiota: community composition, influencing factors, pathogenesis, and interventions. Front Microbiol 13:895537
Li X, Yu D, Wang Y, Yuan H, Ning X, Rui B, Lei Z, Yuan J, Yan J, Li M (2021) The intestinal dysbiosis of mothers with gestational diabetes mellitus (GDM) and its impact on the gut microbiota of their newborns. Can J Infect Dis Med Microbiol 2021:3044534
Li Z, Li Y, Sun Q, Wei J, Li B, Qiu Y, Liu K, Shao D, Ma Z (2022b) Targeting the pulmonary microbiota to fight against respiratory diseases. Cells 11
Lin YP, Chen WC, Cheng CM, Shen CJ (2021) Vaginal pH value for clinical diagnosis and treatment of common vaginitis. Diagnostics (Basel) 11
Liu P, Lu Y, Li R, Chen X (2023) Use of probiotic lactobacilli in the treatment of vaginal infections: In vitro and in vivo investigations. Front Cell Infect Microbiol 13:1153894
Liu Y, Wang Y, Ni Y, Cheung CKY, Lam KSL, Wang Y, Xia Z, Ye D, Guo J, Tse MA (2020) Gut microbiome fermentation determines the efficacy of exercise for diabetes prevention. Cell Metab 31:77–91 e75
Liwinski T, Elinav E (2020) Harnessing the microbiota for therapeutic purposes. Am J Transplant 20:1482–1488
Lloyd-Price J, Abu-Ali G, Huttenhower C (2016) The healthy human microbiome. Genome Med 8:51
Lynch SV, Pedersen O (2016) The human intestinal microbiome in health and disease. N Engl J Med 375:2369–2379
Mac Aogain M, Chandrasekaran R, Lim AYH, Low TB, Tan GL, Hassan T, Ong TH, Hui Qi Ng A, Bertrand D, Koh JY (2018) Immunological corollary of the pulmonary mycobiome in bronchiectasis: the CAMEB study. Eur Respir J 52
Maciel-Fiuza MF, Muller GC, Campos DMS, do Socorro Silva Costa P, Peruzzo J, Bonamigo RR, Veit T, Vianna FSL (2023) Role of gut microbiota in infectious and inflammatory diseases. Front Microbiol 14:1098386
Madan JC, Koestler DC, Stanton BA, Davidson L, Moulton LA, Housman ML, Moore JH, Guill MF, Morrison HG, Sogin ML (2012) Serial analysis of the gut and respiratory microbiome in cystic fibrosis in infancy: interaction between intestinal and respiratory tracts and impact of nutritional exposures. mBio 3
Mahasneh SA, Mahasneh AM (2017) Probiotics: a promising role in dental health. Dent J (Basel) 5
Mahmud MR, Akter S, Tamanna SK, Mazumder L, Esti IZ, Banerjee S, Akter S, Hasan MR, Acharjee M, Hossain MS, Pirttila AM (2022) Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14:2096995
Marchesi JR, Adams DH, Fava F, Hermes GD, Hirschfield GM, Hold G, Quraishi MN, Kinross J, Smidt H, Tuohy KM (2016) The gut microbiota and host health: a new clinical frontier. Gut 65:330–339
Marsh PD (2003) Are dental diseases examples of ecological catastrophes? Microbiology (Reading) 149:279–294
Marsland BJ, Trompette A, Gollwitzer ES (2015) The gut-lung axis in respiratory disease. Ann Am Thorac Soc 2(12 Suppl):S150–S156
Martinez FJ, Yow E, Flaherty KR, Snyder LD, Durheim MT, Wisniewski SR, Sciurba FC, Raghu G, Brooks MM, Kim DY (2021) Effect of antimicrobial therapy on respiratory hospitalization or death in adults with idiopathic pulmonary fibrosis: the CleanUP-IPF randomized clinical trial. JAMA 325:1841–1851
Mayer EA, Knight R, Mazmanian SK, Cryan JF, Tillisch K (2014) Gut microbes and the brain: paradigm shift in neuroscience. J Neurosci 34:15490–15496
Mayer EA, Tillisch K (2011) The brain-gut axis in abdominal pain syndromes. Annu Rev Med 62:381–396
McLaughlin J, Watterson S, Layton AM, Bjourson AJ, Barnard E, McDowell A (2019) Propionibacterium acnes and acne vulgaris: new insights from the integration of population genetic, multi-omic, biochemical and host-microbe studies. Microorganisms 7
Mei Z, Li D (2022) The role of probiotics in vaginal health. Front Cell Infect Microbiol 12:963868
Menees S, Chey W (2018) The gut microbiome and irritable bowel syndrome. F1000Res 7
Mestrovic T, Matijasic M, Peric M, Cipcic Paljetak H, Baresic A, Verbanac D (2020) The role of gut, vaginal, and urinary microbiome in urinary tract infections: from bench to bedside. Diagnostics (Basel) 11
Mortaz E, Adcock IM, Folkerts G, Barnes PJ, Paul Vos A, Garssen J (2013) Probiotics in the management of lung diseases. Mediators Inflamm 2013:751068
Mortaz E, Adcock IM, Ricciardolo FL, Varahram M, Jamaati H, Velayati AA, Folkerts G, Garssen J (2015) Anti-inflammatory effects of Lactobacillus Rahmnosus and Bifidobacterium breve on cigarette smoke activated human macrophages. PLoS One 10:e0136455
Myles IA, Castillo CR, Barbian KD, Kanakabandi K, Virtaneva K, Fitzmeyer E, Paneru M, Otaizo-Carrasquero F, Myers TG, Markowitz TE (2020) Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med 12
Myles IA, Earland NJ, Anderson ED, Moore IN, Kieh MD, Williams KW, Saleem A, Fontecilla NM, Welch PA, Darnell DA (2018) First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight 3
Myles IA, Williams KW, Reckhow JD, Jammeh ML, Pincus NB, Sastalla I, Saleem D, Stone KD, Datta SK (2016) Transplantation of human skin microbiota in models of atopic dermatitis. JCI Insight 1
Nagakubo D, Kaibori Y (2023) Oral microbiota: the influences and interactions of saliva, IgA, and Dietary Factors in Health and Disease. Microorganisms 11
Nakatsuji T, Chen TH, Narala S, Chun KA, Two AM, Yun T, Shafiq F, Kotol PF, Bouslimani A, Melnik AV (2017) Antimicrobials from human skin commensal bacteria protect against Staphylococcus aureus and are deficient in atopic dermatitis. Sci Transl Med 9
Natalini JG, Singh S, Segal LN (2023) The dynamic lung microbiome in health and disease. Nat Rev Microbiol 21:222–235
Navarro-Lopez V, Martinez-Andres A, Ramirez-Bosca A, Ruzafa-Costas B, Nunez-Delegido E, Carrion-Gutierrez MA, Prieto-Merino D, Codoner-Cortes F, Ramon-Vidal D, Genoves-Martinez S (2019) Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: a randomized controlled clinical trial. Acta Derm Venereol 99:1078–1084
O’Callaghan A, van Sinderen D (2016) Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:925
Obuchowska A, Gorczyca K, Standylo A, Obuchowska K, Kimber-Trojnar Z, Wierzchowska-Opoka M, Leszczynska-Gorzelak B (2022) Effects of probiotic supplementation during pregnancy on the future maternal risk of metabolic syndrome. Int J Mol Sci 23
Obuchowska A, Gorczyca K, Standylo A, Obuchowska K, Kimber-Trojnar Z, Wierzchowska-Opoka M, Leszczynska-Gorzelak B Effects of probiotic supplementation during pregnancy on the future maternal risk of metabolic syndrome. Int J Mol Sci 23
Park SH, Lee ES, Park ST, Jeong SY, Yun Y, Kim Y, Jeong Y, Kang CH, Choi HJ (2023) Efficacy and safety of MED-01 probiotics on vaginal health: a 12-week, multicenter, randomized, double-blind, placebo-controlled clinical trial. Nutrients 15
Park SY, Hwang BO, Lim M, Ok SH, Lee SK, Chun KS, Park KK, Hu Y, Chung WY, Song NY (2021) Oral-Gut Microbiome Axis in Gastrointestinal Disease and Cancer. Cancers (Basel) 13
Pellaton C, Nutten S, Thierry AC, Boudousquie C, Barbier N, Blanchard C, Corthesy B, Mercenier A, Spertini F (2012) Intragastric and intranasal administration of Lactobacillus paracasei NCC2461 modulates allergic airway inflammation in mice. Int J Inflam 2012:686739
Peng X, Cheng L, You Y, Tang C, Ren B, Li Y, Xu X, Zhou X (2022) Oral microbiota in human systematic diseases. Int J Oral Sci 14:14
Pessoa R, Clissa PB, Sanabani SS (2023) The interaction between the host genome, epigenome, and the gut-skin axis microbiome in atopic dermatitis. Int J Mol Sci 24
Plaza-Diaz J, Ruiz-Ojeda FJ, Gil-Campos M, Gil A (2018) Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients 10
Prajapati N, Patel J, Singh S, Yadav VK, Joshi C, Patani A, Prajapati D, Sahoo DK, Patel A (2023) Postbiotic production: harnessing the power of microbial metabolites for health applications. Front Microbiol 14:1306192
Qin H, Li G, Xu X, Zhang C, Zhong W, Xu S, Yin Y, Song J (2022) The role of oral microbiome in periodontitis under diabetes mellitus. J Oral Microbiol 14:2078031
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, Liang S, Zhang W, Guan Y, Shen D (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490:55–60
Qu L, Cheng Q, Wang Y, Mu H, Zhang Y (2022) COPD and gut-lung axis: how microbiota and host inflammasome influence COPD and related therapeutics. Front Microbiol 13:868086
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO (2011) Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 1(108 Suppl):4680–4687
Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A 1(108 Suppl):4680–4687
Recine N, Palma E, Domenici L, Giorgini M, Imperiale L, Sassu C, Musella A, Marchetti C, Muzii L, Benedetti Panici P (2016) Restoring vaginal microbiota: biological control of bacterial vaginosis. A prospective case-control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Arch Gynecol Obstet 293:101–107
Reid G, Bruce AW, Fraser N, Heinemann C, Owen J, Henning B (2001) Oral probiotics can resolve urogenital infections. FEMS Immunol Med Microbiol 30:49–52
Rook GA, Raison CL, Lowry CA (2014) Microbial ‘old friends’, immunoregulation and socioeconomic status. Clin Exp Immunol 177:1–12
Round JL, Mazmanian SK (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 9:313–323
Salem I, Ramser A, Isham N, Ghannoum MA (2018) The gut microbiome as a major regulator of the gut-skin axis. Front Microbiol 9:1459
Santos FP, Carvalhos CA, Figueiredo-Dias M (2023) New insights into photobiomodulation of the vaginal microbiome-a critical review. Int J Mol Sci 24
Scherer HU, Haupl T, Burmester GR (2020) The etiology of rheumatoid arthritis. J Autoimmun 110:102400
Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, Li Y, Shen N, Ghedin E, Morris A (2016) Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 1:16031
Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J (2012) Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol 13:R42
Severn MM, Horswill AR (2023) Staphylococcus epidermidis and its dual lifestyle in skin health and infection. Nat Rev Microbiol 21:97–111
Shan Y, Lee M, Chang EB (2022) The gut microbiome and inflammatory bowel diseases. Annu Rev Med 73:455–468
Singh S, Natalini JG, Segal LN (2022) Lung microbial-host interface through the lens of multi-omics. Mucosal Immunol 15:837–845
Skowron K, Bauza-Kaszewska J, Kraszewska Z, Wiktorczyk-Kapischke N, Grudlewska-Buda K, Kwiecinska-Pirog J, Walecka-Zacharska E, Radtke L, Gospodarek-Komkowska E (2021) Human skin microbiome: impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms 9
Slyepchenko A, Maes M, Jacka FN, Kohler CA, Barichello T, McIntyre RS, Berk M, Grande I, Foster JA, Vieta E, Carvalho AF (2017) Gut microbiota, bacterial translocation, and interactions with diet: pathophysiological links between major depressive disorder and non-communicable medical comorbidities. Psychother Psychosom 86:31–46
Sommer F, Backhed F (2013) The gut microbiota--masters of host development and physiology. Nat Rev Microbiol 11:227–238
Suez J, Zmora N, Segal E, Elinav E (2019) The pros, cons, and many unknowns of probiotics. Nat Med 25:716–729
Takada K, Melnikov VG, Kobayashi R, Komine-Aizawa S, Tsuji NM, Hayakawa S (2023) Female reproductive tract-organ axes. Front Immunol 14:1110001
Teughels W, Loozen G, Quirynen M (2011) Do probiotics offer opportunities to manipulate the periodontal oral microbiota? J Clin Periodontol 11(38 Suppl):159–177
Thursby E, Juge N (2017) Introduction to the human gut microbiota. Biochem J 474:1823–1836
Thye AY, Bah YR, Law JW, Tan LT, He YW, Wong SH, Thurairajasingam S, Chan KG, Lee LH, Letchumanan V (2022) Gut-skin axis: unravelling the connection between the gut microbiome and psoriasis. Biomedicines 10
Tilg H, Kaser A (2011) Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 121:2126–2132
Tokarek J, Gadzinowska J, Mlynarska E, Franczyk B, Rysz J (2021) What is the role of gut microbiota in obesity prevalence? A few words about gut microbiota and its association with obesity and related diseases. Microorganisms 10
Tonetti MS, Van Dyke TE, working group 1 of the joint EFPAAPw (2013) Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 84:S24–S29
Trivedi R, Barve K (2020) Gut microbiome a promising target for management of respiratory diseases. Biochem J 477:2679–2696
Tuddenham S, Ghanem KG, Caulfield LE, Rovner AJ, Robinson C, Shivakoti R, Miller R, Burke A, Murphy C, Ravel J, Brotman RM (2019) Associations between dietary micronutrient intake and molecular-bacterial vaginosis. Reprod Health 16:151
Tuniyazi M, Zhang N (2023) Possible therapeutic mechanisms and future perspectives of vaginal microbiota transplantation. Microorganisms 11
Turpin R, Slopen N, Borgogna JC, Yeoman CJ, He X, Miller RS, Klebanoff MA, Ravel J, Brotman RM (2021) Perceived stress and molecular bacterial vaginosis in the national institutes of health longitudinal study of vaginal flora. Am J Epidemiol 190:2374–2383
Twomey KB, Alston M, An SQ, O’Connell OJ, McCarthy Y, Swarbreck D, Febrer M, Dow JM, Plant BJ, Ryan RP (2013) Microbiota and metabolite profiling reveal specific alterations in bacterial community structure and environment in the cystic fibrosis airway during exacerbation. PLoS One 8:e82432
Vasam M, Korutla S, Bohara RA (2023) Acne vulgaris: a review of the pathophysiology, treatment, and recent nanotechnology based advances. Biochem Biophys Rep 36:101578
Versi A, Ivan FX, Abdel-Aziz MI, Bates S, Riley J, Baribaud F, Kermani NZ, Montuschi P, Dahlen SE, Djukanovic R (2023) Haemophilus influenzae and Moraxella catarrhalis in sputum of severe asthma with inflammasome and neutrophil activation. Allergy 78:2906–2920
Villena J, Racedo S, Aguero G, Alvarez S (2006) Yoghurt accelerates the recovery of defence mechanisms against Streptococcus pneumoniae in protein-malnourished mice. Br J Nutr 95:591–602
Wallace CJK, Milev R (2017) The effects of probiotics on depressive symptoms in humans: a systematic review. Ann Gen Psychiatry 16:14
Wang S, Liu Y, Li J, Zhao L, Yan W, Lin B, Guo X, Wei Y (2021a) Fusobacterium nucleatum acts as a pro-carcinogenic bacterium in colorectal cancer: from association to causality. Front Cell Dev Biol 9:710165
Wang X, Zhang P, Zhang X (2021b) Probiotics regulate gut microbiota: an effective method to improve immunity. Molecules 26
Wu J, Peters BA, Dominianni C, Zhang Y, Pei Z, Yang L, Ma Y, Purdue MP, Jacobs EJ, Gapstur SM (2016) Cigarette smoking and the oral microbiome in a large study of American adults. ISME J 10:2435–2446
Wu M, Yang X, Tian J, Fan H, Zhang Y (2021) Antibiotic treatment of pulmonary infections: an umbrella review and evidence map. Front Pharmacol 12:680178
Xie A, Chen A, Chen Y, Luo Z, Jiang S, Chen D, Yu R (2023) Lactobacillus for the treatment and prevention of atopic dermatitis: clinical and experimental evidence. Front Cell Infect Microbiol 13:1137275
Yao Y, Cai X, Ye Y, Wang F, Chen F, Zheng C (2021) The role of microbiota in infant health: from early life to adulthood. Front Immunol 12:708472
Ye C, Kapila Y (2021) Oral microbiome shifts during pregnancy and adverse pregnancy outcomes: Hormonal and Immunologic changes at play. Periodontol 2000 87:276–281
Yeo JM, Lee HJ, Kim JW, Lee JB, Park SY, Choi IS, Song CS (2014) Lactobacillus fermentum CJL-112 protects mice against influenza virus infection by activating T-helper 1 and eliciting a protective immune response. Int Immunopharmacol 18:50–54
Yeoh YK, Zuo T, Lui GC, Zhang F, Liu Q, Li AY, Chung AC, Cheung CP, Tso EY, Fung KS (2021) Gut microbiota composition reflects disease severity and dysfunctional immune responses in patients with COVID-19. Gut 70:698–706
Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI (2020) Gut microbiota and immune system interactions. Microorganisms 8
Yu JC, Khodadadi H, Baban B (2019) Innate immunity and oral microbiome: a personalized, predictive, and preventive approach to the management of oral diseases. EPMA J 10:43–50
Yuksel N, Gelmez B, Yildiz-Pekoz A (2023) Lung microbiota: its relationship to respiratory system diseases and approaches for lung-targeted probiotic bacteria delivery. Mol Pharm 20:3320–3337
Zeldin J, Chaudhary PP, Spathies J, Yadav M, D’Souza BN, Alishahedani ME, Gough P, Matriz J, Ghio AJ, Li Y (2023) Exposure to isocyanates predicts atopic dermatitis prevalence and disrupts therapeutic pathways in commensal bacteria. Sci Adv:eade8898–eade8899
Zhang XE, Zheng P, Ye SZ, Ma X, Liu E, Pang YB, He QY, Zhang YX, Li WQ, Zeng JH, Guo J (2024) Microbiome: role in inflammatory skin diseases. J Inflamm Res 17:1057–1082
Zhang Y, Ding Y, Guo Q (2022) Probiotic species in the management of periodontal diseases: an overview. Front Cell Infect Microbiol 12:806463
Zhong X, Lu Q, Zhang Q, He Y, Wei W, Wang Y (2021) Oral microbiota alteration associated with oral cancer and areca chewing. Oral Dis 27:226–239
Acknowledgements
This work was supported by the Division of Intramural Research (DIR) of the National Institute of Allergy and Infectious Diseases, NIH.
Funding
Open access funding provided by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chaudhary, P.P., Kaur, M. & Myles, I.A. Does “all disease begin in the gut”? The gut-organ cross talk in the microbiome. Appl Microbiol Biotechnol 108, 339 (2024). https://doi.org/10.1007/s00253-024-13180-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-024-13180-9