Abstract
Fungal infections are increasing rapidly, and antifungal agents used in clinics are limited. Therefore, novel antifungal agents with high efficiency are urgently required. In this study, we investigated the antifungal activity of thonningianin A (THA), a natural compound that is widely found in plants. We first determined the activity of THA against Candida albicans, one of the most common fungal pathogens, and found that THA showed antifungal activity against all C. albicans tested, including several fluconazole-resistant isolates. THA also inhibits the growth of non-Candida albicans species. In addition, THA displayed antibiofilm activity and could not only inhibit biofilm formation but also destroy mature biofilms. The in vivo antifungal efficacy of THA was confirmed in a Galleria mellonella infection model. Further studies revealed that THA could enhance intracellular reactive oxygen species (ROS) production and regulate the transcription of several redox-related genes. Specifically, caspase activity and expression of CaMCA1, a caspase-encoding gene in C. albicans, were remarkably increased upon THA treatment. Consistent with this, in the presence of THA, the Camca1 null mutant displayed higher survival rates and reduced caspase activity compared to the wild-type or CaMCA1-reintroduced strains, indicating an important role of CaMCA1 in the antifungal activity of THA. Taken together, our results indicate that THA possesses excellent antifungal activity and may be a promising novel antifungal candidate.
Key points
• THA exhibits activity against Candida species, including fluconazole-resistant isolates
• THA inhibits biofilm formation and destroys mature biofilm
• Elevated ROS production and CaMCA1-mediated caspase activity are involved in the antifungal mechanisms of THA
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fungal infections have become increasingly common in recent years, especially in patients with immune systems weakened by cancer, infection with human immunodeficiency virus, or administration of immunosuppressive drugs (Pfaller and Castanheira 2016; Chang et al. 2017; Benedict et al. 2019). Candida albicans is one of the most common pathogens of fungal infection. When the human immune defenses become impaired, this fungus can be a common cause of superficial, mucosal, and systemic infections (Wang 2015). Unlike antibacterial drugs, clinical antifungal drugs are limited. Azoles (such as fluconazole), polyenes (such as amphotericin B), and echinocandins (such as caspofungin) are the three main types of drugs used in clinics against fungal species (Somer et al. 2011; Prasad et al. 2016; Wiederhold 2017). However, the disadvantages of these drugs often limit their wide clinical application. For example, amphotericin B can cause severe side effects such as fever, nausea, and vomiting (Laniado-Laborín and Cabrales-Vargas 2009). In addition, the antifungal spectrum of echinocandins is relatively narrow and the economic burden for the patients with echinocandins administration is much higher as compared to several other commonly used. Furthermore, the administration of azoles has resulted in drug resistance, and this is common with drugs such as fluconazole (FLC) antifungals. Due to its broad antifungal spectrum and low toxicities, fluconazole has a wide clinical application. However, long-term use of this drug inevitably leads to drug resistance, and fluconazole-resistant isolates are constantly emerging, which is a serious problem for antifungal therapy (Allen et al. 2015; Azevedo et al. 2015). Thus, there is an urgent need to develop novel antifungal agents, and the potential antifungal activities of plant-derived natural compounds are receiving increasing attention due to their extensive supply sources and easy accessibility. Many research groups have reported the activities of natural compounds against C. albicans and other fungal pathogens. For example, bacalein exhibited antifungal activity against C. albicans and non-Candida spp. (Kang et al. 2010; Lu et al. 2017; Aldholmi et al. 2019). Shikonin showed activity against both planktonic and biofilm growth of C. albicans (Yan et al. 2019). Other natural compounds that have been reported to exhibit antifungal activities include allicin, pterostilbene, roemerine, and so on (Khodavandi et al. 2010; Li et al. 2014; Ma et al. 2015). Thonningianin A (THA, Fig. 1) is an ellagic tannin flavonoid widely found in natural plants. Previous studies have revealed that THA possesses multiple biological activities, including antioxidant, antiproliferative, and anticancer activities (Gyamfi and Aniya 2002; Gyamfi et al. 2004; Lu et al. 2012; Huang et al. 2014; Zhang et al. 2015). Recent studies showed that THA could enhance microglial autophagy via the AMPK/ULK1 and Raf/MEK/ERK pathways; thus, this compound is effective in the therapy of Alzheimer’s disease (Huang et al. 2014; Zhou et al. 2022). In addition, THA was found to ameliorate vascular calcification in type 2 diabetes mellitus via the activation of L-type calcium ion channels (Shen et al. 2023). In this study, we aimed to investigate the antifungal activity of THA and explore its potential mechanisms of action.
Materials and methods
Strains, chemicals, and medium
The fungi used in this study included the C. albicans standard strain SC5314, CaMCA1-deleted (Camca1△/Camca1△), reintroduced (CaMCA1-EXP), and the corresponding wild-type strains (CAF2-1) (Cao et al. 2009). Other Candida species used in the experiments included the clinical fungal isolates of C. albicans, C. parapsilosis, C. tropicalis, Nakaseomyces glabrata (previous name Candida glabrata), and Pichia kudriavzevii (previous name Candida krusei). These strains were obtained from Shanghai Changhai Hospital and Shanghai Skin Disease Hospital (China) and were isolated from patients with invasive, mucosal, and superficial fungal infections. Fungal cells were routinely cultured in yeast peptone dextrose (YPD) medium. RPMI 1640 (Gibco, USA) was used to determine the minimum inhibitory concentrations (MICs) and antibiofilm activity of the drugs. FLC and THA were purchased from Macklin Biochemical Company (Shanghai, China) and Sigma-Aldrich Company (USA), respectively.
Determination of minimum inhibitory concentrations
The MICs of THA and FLC were determined using the broth microdilution method as described previously with some modifications (Clinical & Laboratory Standards Institute 2017). The overnight-grown fungal cells were harvested and adjusted to 2 × 103 cells/ml in RPMI 1640 medium. The concentrations of the drugs added to the plates ranged from 0.125 to 64 μg/ml. The plates were incubated at 35°C for 24 to 48 h. The optical density was measured by a microplate reader at 630 nm (OD630). The MIC50 and MIC90 values for the drugs were defined as 50 and 90% growth inhibition in the drug-treated groups as compared to the drug-free (control) group, respectively.
Growth curve assay
The growth curve assay was performed as previously described (Quan et al. 2006). Briefly, C. albicans SC5314 or isolate 332 cells grown to exponential phase were diluted with RPMI 1640 medium to approximately 1 × 103 cells/ml. The fungal cells were then exposed to different concentrations of THA or FLC and cultured at 30°C for 24 h with vigorous shaking (over 200 rpm). At each indicated time point, 10 μl of the culture was collected from each group, diluted, and incubated at 30°C for 48 h. The colonies were counted under a microscope and converted into log10 CFU/ml.
Antibiofilm assay
The antibiofilm ability of THA was evaluated as previously described (Pierce et al. 2008). Briefly, the overnight-grown C. albicans SC5314 cells were adjusted to 1.0 × 106 cells/ml in RPMI 1640 medium and added to 96-well tissue culture plates. Following the initial 90-min adhesion at 37°C the medium was aspirated, and fresh medium was added. The plates were further incubated at 37°C for 24 h. To monitor the effects of THA on biofilm formation, different concentrations of THA (ranging from 2 to 32 μg/ml) were added after 90 min of adhesion. To monitor the effect of THA on mature biofilms, the biofilms were allowed to form for 24 h, and fresh RPMI 1640 medium containing different concentrations of THA was added. The plates were then incubated at 37°C for an additional 24 h.
XTT reduction assay
The antibiofilm activity of THA was measured using the XTT reduction assay, a reaction catalyzed by mitochondrial dehydrogenases (Ramage et al. 2001). Briefly, the grown biofilms were washed and treated with 0.5 mg/ml XTT and 1 mM menadione for 90 min at 37°C, then measured at 490 nm using a microtiter plate reader.
Assessment of biofilm biomass
Biofilm biomass was assessed as previously described with some modifications (Nobile et al. 2006). The silicone disks (1.5 × 1.5 cm, Bentec Medical Corp., USA) used for determining the biofilm biomass were carefully weighed. To prepare for the biofilm biomass assay, disks were treated with bovine serum (Gibco, USA) at 37°C overnight and washed with PBS. After the pretreatment above, the disks were placed into 12-well tissue culture plates with one disk in each well. Next, the adjusted cell suspension (1.0 × 106 cells/ml of overnight grown C. albicans SC5314 cells) was added to the plates. After 90 min of adhesion, a new RPMI 1640 medium containing THA was added to the well. The plate was further incubated at 37°C for 24 h with gentle agitation to allow for biofilm formation. The silicone disks were then collected and dried at room temperature to a constant weight. The biomass value was obtained by subtracting the mass of the empty silicone disk from that of the biofilm-grown silicone disk and further adjusting to obtain the silicone square weight.
Scanning electron microscopy analysis
To observe the effect of THA on C. albicans biofilm formation with scanning electron microscopy (SEM), we developed C. albicans biofilms on silicon disks, as described above. THA was added to the wells containing the disks after 90 min of adhesion. The plate was further incubated at 37°C for 24 h with gentle agitation to allow for biofilm formation. The disks were then collected, washed three times with PBS, and fixed with 3% glutaraldehyde. After this, the disks were washed with 0.1 M Na3PO4 buffer (pH 7.2), dehydrated in ethanol, and coated with gold. The biofilms were observed by an environment SEM.
Galleria mellonella assay
An in vivo killing assay using Galleria mellonella (G. mellonella) as the infection model was performed as previously described (Li et al. 2013). C. albicans SC5314 cells grown overnight were collected, washed, and adjusted to 1 × 108 cells/ml. Each larva (approximately 300 mg) was then injected at the last left pro-leg with 5 μl of the cell suspension (containing 5 × 105 cells). THA was delivered to the larvae 30 min after injection of the fungal cells. The death of the larvae was counted daily for 10 days. For the fungal burden assay, five larvae were homogenized 1 day after drug administration. The homogenate was incubated on YPD at 30°C for 2 days, and the log reduction in CFU/larva was calculated.
Assessment of reactive oxygen species
Intracellular reactive oxygen species (ROS) production was assessed as previously described (Li et al. 2017). Overnight-grown C. albicans SC5314 cells were adjusted to 1 × 107 cells/ml. Next, 20 μg/ml DCFH-DA (Molecular Probes, USA) was added, and the cells were cultured with constant shaking (200 rpm) at 30°C for 30 min. Subsequently, the cells were washed and treated with THA. At each indicated time point, the fluorescence values of the cells were determined using a multiple-mode microplate reader (Synergy H1, USA) with excitation at 485 nm and emission at 520 nm.
Reverse transcription-quantitative PCR
The reverse transcription quantitative PCR (RT-qPCR) was performed as previously described (Yan et al. 2019). Briefly, overnight grown C. albicans SC5314 cells were washed and transferred to a fresh RPMI 1640 medium (1:100 dilution) and cultured with constant shaking (200 rpm) at 30°C for 4 h. The cells were then treated with THA (2, 4, or 8 μg/ml) and continually cultured under the same condition for 1, 2, or 4 h. For detecting the effect of THA on the gene expression in biofilms, overnight-grown C. albicans SC5314 cells were adjusted to 1.0 × 106 cells/ml in RPMI 1640 medium and added to tissue culture plates. After initial 90 min of adhesion, the medium was aspirated, and a fresh medium containing 8 μg/ml THA was added. The plates were further incubated at 37°C for 24 h. Total RNA was extracted using a fungal RNAout kit (TIANZ, Beijing, China). Experiments were performed with the Light Cycler System (Roche Diagnostics, GmbH Mannheim, Germany). The PCR protocol consisted of an initial step at 95°C for 2 min, followed by 40 cycles of amplification and quantification (95°C for 10 s, 60°C for 20 s, 72°C for 15 s), and finally a cooling step to 40°C. The primer sequences for the genes detected are listed in Table 1. The mRNA levels were normalized to their 18S rRNA levels.
Caspase activity assay
For the determination of caspase activity, overnight-grown C. albicans SC5314 cells were washed and transferred to a fresh RPMI 1640 medium by 1:100 and cultured with constant shaking (200 rpm) at 30°C for 4 h. Subsequently, the cells were exposed to different concentrations of THA (4, 8, and 16 μg/ml) and continually cultured under the same condition for a certain time (3, 6, 12, and 24 h). Caspase activity was assayed by staining the C. albicans cells with D2R (CaspScreen flow cytometric apoptosis detection kit, BioVision), which could be cleaved into green fluorescent rhodamine 110 (Hao et al. 2013). Briefly, C. albicans cells treated with THA were washed and incubated with D2R at 30°C for 45 min before viewing and counting under a fluorescence microscope with excitation at 485 nm and emission at 520 nm.
Statistical criteria
The results are presented as means and standard deviations. The data were analyzed using GraphPad Prism 6.0. A P value of < 0.05 or < 0.01 was considered statistically significant. The MIC data of the drugs were defined as 50 or 90% growth inhibition of the strains tested by comparing OD630 values of the drug-treated group with the drug-free (control) group.
Results
Antifungal activity of THA against C. albicans
First, the activity of THA, which is an ellagic tannin flavonoid compound widely found in plants, against C. albicans was determined. The tested strains contained the widely used standard C. albicans strain SC5314 and several clinical isolates (including two fluconazole-sensitive and five fluconazole-resistant isolates obtained from patients with invasive, mucosal, or superficial fungal infections). The MIC90 value of THA against the C. albicans strains ranged from 2 to 8 μg/ml (Table 2). In the growth curve assay, THA at a dose of 8 μg/ml could remarkably inhibit the growth of SC5314 cells, with an approximately 1 × 104 CFU/ml decrease being observed as compared to the control (drug-free) group at the time point of 12 h. Treatment with 16 μg/ml of THA resulted in a dramatic drop in the growth curve during the whole course of treatment (Fig. 2A). Moreover, THA possessed antifungal activity against fluconazole-resistant C. albicans isolates (such as isolates 589, 332, and 265) similar to that of fluconazole-sensitive isolates (such as isolates Y0109 and 638), with MIC90 values over 8- or 16-fold lower than that of fluconazole. For example, the MIC90 values of THA and fluconazole against the fluconazole-resistant C. albicans isolate 332 were 4 and > 64 μg/ml, respectively. The growth curve assay showed that treatment with 64 μg/ml fluconazole had no obvious growth inhibition on isolate 332, whereas the presence of THA exhibited dose-dependent antifungal activities. After treatment with 16 μg/ml THA for 12 h, the growth curve approached the x-axis, indicating strong antifungal activity (Fig. 2B).
The growth curves were obtained by incubating C. albicans strain SC5314 (A) and fluconazole-resistant isolate 332 (B) with THA or/and fluconazole. The Log10 of CFU/ml remaining of the fungal cells upon exposure to 4, 8, and 16 μg/ml THA or/and 64 μg/ml fluconazole (FLC) were counted. Data are shown as the mean ± standard deviation of the independent assays in triplicate. *P < 0.05; **P < 0.01 as compared to the THA-free group
Activity of THA against non-albicans Candida species
We next tested whether THA possessed antifungal activity against non-albicans Candida species. The MIC values of THA against C. parapsilosis, C. tropicalis, Nakaseomyces glabrata, and Pichia kudriavzevii were determined. As shown in Table 2, all non-albicans Candida species isolates tested were sensitive to THA treatment. The MIC90 values of THA against these isolates ranged from 2 to 8 μg/ml. It should be noted that, consistent with reports from other researchers (Beardsley et al. 2018), many non-albicans Candida isolates were less susceptible to fluconazole. Specifically, all Nakaseomyces glabrata and Pichia kudriavzevii isolates in this study were resistant to fluconazole. Thus, the similar MIC90 values of THA against C. albicans and non-albicans Candida species indicated a broad antifungal property of THA.
Effect of THA on C. albicans biofilm
The ability to form biofilms is an important virulence factor for C. albicans, often resulting in its high resistance to antifungal agents (Finkel and Mitchell 2011). Here, the antibiofilm activity of THA was determined. THA exhibited inhibitory activity on biofilm formation in a dose-dependent manner. The presence of 2 μg/ml THA displayed a weak but significant inhibition (P < 0.05) on biofilm formation, whereas 8 μg/ml THA showed a dramatic inhibition on biofilm formation. When 32 μg/ml THA was added, the biofilm formation was almost completely inhibited (Fig. 3A). Moreover, THA exhibited activity against mature biofilms. Upon exposure to 8 μg/ml THA, the growth of mature biofilm was significantly inhibited, and 32 μg/ml THA led to an even more severe impact on the mature biofilm (Fig. 3B).
Antibiofilm activities of THA against C. albicans SC5314 biofilm detected by XTT reduction assay. A Effects of THA on biofilm formation. B Effects of THA on mature biofilms. C Effects of THA on the biomass production of the biofilms. The results were presented as the percent of THA-treated biofilms relative to the THA-free (control) biofilms. D RT-qPCR was used to detect the mRNA levels of the C. albicans genes when 8 μg/ml THA was used to inhibit biofilm formation for 24 h. The transcription of the genes was shown as the fold change in the THA-treated group relative to that of the THA-free group. E Effects of 16 μg/ml THA on biofilm formation, shown in SEM images. Data are shown as the mean ± standard deviation of the independent assays in triplicate. *P < 0.05; **P < 0.01 as compared to the THA-free group
To further confirm the antibiofilm effect of THA, we determined biofilm biomass upon exposure to THA. Consistent with the results obtained from the XTT reduction assay, THA could affect the production of biofilm biomass (Fig. 3C). Treatment with 4 μg/ml THA showed a remarkable influence on the production of biomass. The decrease in biomass production was even more obvious when the THA concentration was increased, and the addition of 32 μg/ml THA led to an approximately 10-fold drop in the biofilm biomass as compared to the drug-free biofilm. In addition, RT-qPCR revealed that THA could affect the expression of several genes involved in biofilm formation. As shown in Fig. 3D, when the biofilms were inhibited by 8 μg/ml THA for 24 h, the transcription levels of EFG1, ECE1, ALS1, ALS3, and HWP1 were remarkably downregulated, while the expression of RAS1 was almost not affected.
The effect of THA on biofilm was further detected with SEM. As shown in Fig. 3E, the biofilm formed by C. albicans SC5314 without THA treatment exhibited a three-dimensional structure and was mainly composed of long hyphae. The presence of 16 μg/ml THA seriously influenced the structure of the biofilm, which was composed of yeast cells, pseudohyphae, and less dense hyphae.
Antifungal activity of THA in vivo
The in vivo activity of THA against C. albicans was determined using a G. mellonella infection model. All G. mellonella infected with C. albicans died within four days when no drug was administered (Fig. 4A). Administration of 5 mg/kg THA resulted in an obviously prolonged survival period with G. mellonella alive for 10 days after infection. When 10 mg/kg THA was administered, the mortality rate of G. mellonella remarkably dropped, with approximately 50% of G. mellonella living for more than 10 days.
A The survival of G. mellonella larvae (n = 15) infected with C. albicans SC5314 cells and treated with THA (5 and 10 mg/kg) 30 min after infection. B The fungal burden of the larvae (n = 5) infected with C. albicans SC5314 cells and treated with THA (5 and 10 mg/kg) 1 day after infection. Statistical significance among the groups was analyzed by one-way ANOVA. *P < 0.05; **P < 0.01 as compared to the THA-free group
We further investigated the in vivo activity of THA using fungal burden analysis. In the absence of THA treatment, a high fungal burden was observed in the C. albicans-infected G. mellonella (Fig. 4B). Administration of 5 mg/kg THA led to a significant decrease in fungal burden. Treatment with 10 mg/kg THA caused an even more obvious decrease in fungal burden, with an approximately 100-fold reduction as compared to the drug-free group.
THA induces intracellular ROS production
Since oxidative damage caused by ROS is an important mechanism of action for antifungal agents (Ghannoum and Rice 1999; Kobayashi et al. 2002; Zhao et al. 2016), we tested the effect of THA on intracellular ROS production. A remarkable ROS elevation was observed in C. albicans cells upon exposure to THA (Fig. 5A). The addition of 4 μg/ml THA significantly stimulated the production of intracellular ROS. The presence of 16 μg/ml THA led to rapid and strong ROS production. At the time point of 6 h, the ROS levels were approximately three times higher in the 16 μg/ml THA group than in the 4-μg/ml THA group.
A THA increased intracellular ROS production. C. albicans SC5314 cells were exposed to 4, 8, and 16 μg/ml THA, respectively. B THA affected the transcription of the genes involved in oxidative stress. The C. albicans SC5314 cells were exposed to 4 μg/ml THA for 4 h. The transcription of the genes is shown as the fold change in the THA-treated group relative to that of the THA-free group. Data are shown as the mean ± standard deviation of the independent assays in triplicate. *P < 0.05; **P < 0.01 as compared to the THA-free group
Next, we evaluated the mRNA levels of several redox-related genes in C. albicans using RT-qPCR. The addition of THA resulted in the upregulation of GRP2 (a NADPH-dependent methylglyoxal reductase, 3.6-fold increase), GLR1 (a glutathione reductase gene, 2.4-fold increase), CAP1 (a transcription factor specific to the oxidative stress response, 2.3-fold increase), and TRR1 (a thioredoxin reductase gene, 2.1-fold increase). However, the expression of two manganese superoxide dismutases, SOD2 and SOD5, was downregulated by approximately 3- and 2-fold, respectively (Fig. 5B).
THA enhances caspase activity and CaMCA1 expression
In C. albicans, increased intracellular ROS levels are closely related to high caspase activity (Al-Dhaheri and Douglas 2010; Lu et al. 2011). Thus, the caspase activity in C. albicans cells was evaluated by staining the cells with the fluorescent dye D2R. As shown in Fig. 6A, the addition of THA resulted in a remarkable impact on caspase activity. At the time point of 24 h, 4 and 8 μg/ml THA increased caspase activity by 2.4- and 4.7-fold (P < 0.01), respectively. When the fungal cells were treated with 16 μg/ml THA for 24 h, the caspase activity increased by approximately 6 times compared to that in the control (drug-free) group.
Effect of THA on the caspase activity and CaMCA1 expression. A C. albicans SC5314 cells were exposed to 4, 8, and 16 μg/ml THA, respectively. At the time points of 3, 6, 12, and 24 h, the caspase activity was detected. B THA affected the transcription of CaMCA1. The C. albicans SC5314 cells were exposed to 2, 4, and 8 μg/ml THA, respectively. The transcription of CaMCA1 was detected at the time points of 1, 2, and 4 h and shown as the fold change in the THA-treated group relative to that of the THA-free group. Data are shown as the mean ± standard deviation of the independent assays in triplicate. *P < 0.05; **P < 0.01 as compared to the time point of 0 h
The expression of CaMCA1, which encodes a metacaspase and is responsible for caspase activity in C. albicans, was analyzed using RT-qPCR. Consistent with the stimulation of caspase activity by THA, expression of CaMCA1 was enhanced upon THA treatment. Although 2 and 4 μg/ml THA showed a slight effect on the level of CaMCA1 mRNA at the time point of 1 h (1.37- and 1.57-fold increase, respectively), the presence of 8 μg/ml THA significantly upregulated the mRNA level of CaMCA1 at this time point (2.5-fold increase). When the fungal cells were exposed to 8 μg/ml THA for 4 h, the level of CaMCA1 mRNA increased by 3.6-fold compared to that in the control group (Fig. 6B).
The Camca1 mutant shows resistance to THA
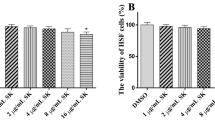
In view of the enhanced transcription of CaMCA1 in C. albicans cells upon THA treatment, we evaluated the role of Camca1 in the antifungal effect of THA. As shown in Fig. 7A, there was an obvious difference in the survival rates between the Camca1 mutant (Camca1△/Camca1△) and wild-type cells (CAF2-1) upon THA treatment. When 4 μg/ml THA was added, the survival rates of the wild-type, Camca1 mutant, and CaMCA1-reintroduced (CaMCA1-EXP) strains were 75, 91, and 73%, respectively. Consistently, the Camca1 mutant displayed lower caspase activity than the wild-type and CaMCA1-reintroduced cells (Fig. 7B). The effect of CaMCA1 deletion on the cell sensitivity to 16 μg/ml THA was even more obvious, as the survival rate of the Camca1 mutant (48%) was approximately 7 times higher than that of the wild-type cells (7%). Consistent with this, the caspase activity in the wild-type, Camca1 mutant, and CaMCA1-reintroduced strains was 66, 17, and 62%, respectively, in the 16-μg/ml THA-treated group.
A Effect of CaMCA1 on THA-induced cell death. The C. albicans Camca1 mutant (Camca1△/Camca1△), CaMCA1-reintroduced (CaMCA1-EXP), and wild-type (CAF2-1) strains were exposed to 4, 8, and 16 μg/ml THA for 12 h. The cells were then washed twice with PBS and plated on YPD agar plates. The fraction of viable cells was determined by counting the colonies and calculating the percentage of survived fungal cells relative to the control drug-free cells. B Effect of CaMCA1 on THA-induced caspase activity. The C. albicans strains were exposed to 4, 8, and 16 μg/ml THA for 12 h, and then the caspase activity was detected. Data are shown as the mean ± standard deviation of the independent assays in triplicate. *P < 0.05; **P< 0.01 compared with values from the control wild-type cells
Discussion
Currently, fungal infections are common and have become a serious threat to immunocompromised patients (Jenks et al. 2020). Unlike a wide variety of antibacterial drugs, antifungal drugs are limited and often have disadvantages, such as unbearable adverse drug reactions, ineffectiveness against drug-resistant strains, and high cost, which often results in the premature termination of antifungal therapy. Our results showed that THA, a natural compound widely present in plants, possessed potent activities against C. albicans. Moreover, THA displayed inhibitory effects on C. albicans isolates resistant to fluconazole, which is one of the most commonly used antifungal agents, resulting in the rapid emergence of resistant isolates. The antifungal activities of THA were further confirmed by a growth curve assay, in which THA exhibited a dose- and time-dependent manner against both fluconazole-sensitive and fluconazole-resistant C. albicans strains. These results suggest that THA is effective against resistant C. albicans and may possess a mechanism of action different from that of fluconazole. In addition, THA showed activity against a series of non-Candida spp., including C. parapsilosis, C. tropicalis, Nakaseomyces glabrata, and Pichia kudriavzevii, indicating a broad antifungal spectrum.
One of the major virulence factors of C. albicans is its ability to form a biofilm, which is a group lifestyle of microorganisms with high resistance to various antifungal agents, including fluconazole, amphotericin B, and caspofungin (Chandra et al. 2001). Our results showed that THA not only inhibited biofilm formation but also destroyed the maintenance of mature biofilms. The addition of 32 μg/ml THA almost completely inhibited biofilm formation, whereas over 70% of the mature biofilm was destroyed at this THA concentration. In addition, the transcription of EFG1 (a transcription regulator controlling filamentous growth), HWP1 and ECE1 (both mainly expressed on the hyphal surface), and ALS1 and ALS3 (two adhesin genes) was remarkably inhibited (Sundstrom 2002; Nobile et al. 2006; Araújo et al. 2017). Since hyphae growth and adhesion are important for biofilm formation, the downregulation of these genes was consistent with the inhibitory effect of THA on biofilm formation. These results indicate that THA may be a potent antifungal agent for the treatment of various forms of clinical fungal infections, such as biofilm-related infections.
Induction of intracellular ROS production in fungi is an important mechanism of action for antifungal agents, such as amphoterin B, caspofungin, and miconazole. Accumulated ROS have strong oxidant activity and can attack large molecules, such as DNA, proteins, and nucleic acids, causing irreversible damage to fungal cells (Sokol-Anderson et al. 1986; Fernández-García et al. 2022). In this study, a striking increase in the level of intracellular ROS was detected in THA-treated C. albicans cells. Consistent with this, a series of redox-related genes were found to be differentially expressed upon THA treatment. As a transcription factor in C. albicans, CAP1 can aggregate in the nucleus and regulate the transcription of many redox-related genes (Wang et al. 2006). Here, the increased expression of CAP1 in the THA-treated group might be due to the altered harmful intracellular environment, in which the fungal cells need to activate this transcription factor to upregulate the expression of specific downstream antioxidant defense-related genes; thus, an efficient oxidative stress response is initiated (Alarco and Raymond 1999; Zhang et al. 2000). Consistently, our results revealed that GRP2, GLR1, and TRR1, which are CAP1-responsive genes with antioxidant scavenging/defense properties and encode NADPH-dependent methylglyoxal reductase, glutathione reductase, and TRR1 thioredoxin reductase, respectively, were upregulated. The increased expression of these genes might be considered negative feedback in response to THA-induced ROS accumulation. SOD2 and SOD5 are two superoxide dismutases that catalyze the direct removal of ROS and play critical roles in the first line of defense against antioxidants (Tyler 1975; Luk et al. 2005). It was reported that C. albicans cells lacking superoxide dismutase are hypersensitive to oxidative stress (Hwang et al. 2003). Therefore, the reduced expression of SOD2 and SOD5 in fungal cells upon exposure to THA might further promote the accumulation of intracellular ROS and the corresponding oxidative damage, which leads to cell death.
Previous studies have shown that enhanced ROS production is closely related to high caspase activity (Cao et al. 2009). In the current study, caspase activity in C. albicans upon THA treatment was remarkably increased. The mRNA level of CaMCA1, which encodes the only known metacaspase in C. albicans, increased in the THA-treated groups. This result is consistent with previous reports, which showed that, as a homolog of Saccharomyces cerevisiae metacaspase YCA1, overexpression of CaMCA1 in C. albicans is closely related to ROS accumulation and caspase activation. Upon H2O2-induced oxidative damage, the Camca1 mutant displayed higher viability and lower caspase activity as compared to the wild-type strain (Cao et al. 2009; Jung and Kim 2014). To further investigate the role of CaMCA1 in THA-induced cell death, we tested whether the presence of CaMCA1 affects the sensitivity of C. albicans cells to THA killing. Our results showed that, compared to the wild-type and CaMCA1-reintroduced strains, the survival rate of the Camca1 mutant was significantly higher, accompanied by lower caspase activity. This result indicates an important role of CaMCA1 in the antifungal activity of THA.
In conclusion, this study demonstrated the activity of the natural compound THA in vitro and in vivo, which exhibited highly efficient and broad-spectrum antifungal activities. Further studies revealed that elevated intracellular ROS production and CaMCA1-mediated high caspase activity might be involved in the mechanisms underlying the antifungal action of THA.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Alarco AM, Raymond M (1999) The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J Bacteriol 181:700–708
Al-Dhaheri RS, Douglas LJ (2010) Apoptosis in Candida biofilms exposed to amphotericin B. J Med Microbiol 59:149–157
Aldholmi M, Marchand P, Ourliac-Garnier I, Le Pape P, Ganesan A (2019) A decade of antifungal leads from natural products: 2010-2019. Pharmaceuticals (Basel) 12:182
Allen D, Wilson D, Drew R, Perfect J (2015) Azole antifungals: 35years of invasive fungal infection management. Expert Rev Anti Infect Ther 13:787–798
Araújo D, Henriques M, Silva S (2017) Portrait of Candida species biofilm regulatory network genes. Trends Microbiol 25:62–75
Azevedo MM, Faria-Ramos I, Cruz LC, Pina-Vaz C, Rodrigues AG (2015) Genesis of azole antifungal resistance from agriculture toclinical settings. J Agric Food Chem 63:7463–7468
Beardsley J, Halliday CL, Chen SC, Sorrell TC (2018) Responding to the emergence of antifungal drug resistance: perspectives from the bench and the bedside. Future Microbiol 13:1175–1191
Benedict K, Jackson BR, Chiller T, Beer KD (2019) Estimation of direct healthcare costs of fungal diseases in the United States. Clin Infect Dis 68:1791–1797
Cao YY, Huang S, Dai BD, Zhu ZY, Lu H, Dong LL, Cao YB, Wang Y, Gao PH, Chai YF, Jiang YY (2009) Candida albicans cells lacking CaMCA1-encoded metacaspase show resistance to oxidative stress-induced death and change in energy metabolism. Fungal Genet Biol 46:183–189
Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA (2001) Antifungal resistance of candida biofilms formed on denture acrylic in vitro. J Dent Res 80:903–908
Chang YL, Yu SJ, Heitman J, Wellington M, Chen YL (2017) New facets of antifungal therapy. Virulence 8:222–236
Clinical & Laboratory Standards Institute (2017) M27-A4: reference method for broth dilution antifungal susceptibility testing of yeasts, 4th edn. Wayne, PA, USA, CLSI
Fernández-García R, Muñoz-García JC, Wallace M, Fabian L, González-Burgos E, Gómez-Serranillos MP, Raposo R, Bolás-Fernández F, Ballesteros MP, Healy AM, Khimyak YZ, Serrano DR (2022) Self-assembling, supramolecular chemistry and pharmacology of amphotericin B: poly-aggregates, oligomers and monomers. J Control Release 341:716–732
Finkel JS, Mitchell AP (2011) Genetic control of Candida albicans biofilm development. Nat Rev Microbiol 9:109–118
Ghannoum MA, Rice LB (1999) Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev 12:501–517
Gyamfi MA, Aniya Y (2002) Antioxidant properties of Thonningianin A, isolated from the African medicinal herb Thonningia sanguine. Biochem Pharmacol 63:1725–1737
Gyamfi MA, Ohtani II, Shinno E, Aniya Y (2004) Inhibition of glutathione S-transferases by thonningianin A, isolated from the African medicinal herb, Thonningiasan guinea, in vitro. Food Chem Toxicol 42:1401–1408
Hao BH, Cheng SJ, Clancy CJ, Nguyen MH (2013) Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob Agents Chemother 57:326–332
Huang DD, Jiang Y, Chen WS, Yao FY, Sun LN (2014) Polyphenols with anti-proliferative activities from penthorum Chinense Pursh. Molecules 19:11045–11055
Hwang CS, Baek YU, Yim HS, Kang SO (2003) Protective roles of mitochondrial manganese-containing superoxide dismutase against various stresses in Candida albicans. Yeast 20:929–941
Jenks JD, Cornely OA, Chen SC, Thompson GR 3rd, Hoenigl M (2020) Breakthrough invasive fungal infections: who is at risk? Mycoses 63:1021–1032
Jung JH, Kim J (2014) Roles of Edc3 in the oxidative stress response and CaMCA1-encoded metacaspase expression in Candida albicans. FEBS J 281:4841–4851
Kang K, Fong WP, Tsang PW (2010) Antifungal activity of baicalein against Candida krusei does not involve apoptosis. Mycopathologia 170:391–396
Khodavandi A, Alizadeh F, Aala F, Sekawi Z, Chong PP (2010) In vitro investigation of antifungal activity of allicin alone and in combination with azoles against Candida species. Mycopathologia 169:287–295
Kobayashi D, Kondo K, Uehara N, Otokozawa S, Tsuji N, Yagihashi A, Watanabe N (2002) Endogenous reactive oxygen species is an important mediator of miconazole antifungal effect. Antimicrob Agents Chemother 46:3113–3117
Laniado-Laborín R, Cabrales-Vargas MN (2009) Amphotericin B: side effects and toxicity. Rev Iberoam Micol 26:223–227
Li DD, Deng L, Hu GH, Zhao LX, Hu DD, Jiang YY, Wang Y (2013) Using Galleria mellonella-Candida albicans infection model to evaluate antifungal agents. Biol Pharm Bull 36:1482–1487
Li DD, Zhao LX, Mylonakis E, Hu GH, Zou Y, Huang TK, Yan L, Wang Y, Jiang YY (2014) In vitro and in vivo activities of pterostilbene against Candida albicans biofilms. Antimicrob Agents Chemother 58:2344–2355
Li DD, Chai D, Huang XW, Guan SX, Du J, Zhang HY, Sun Y, Jiang YY (2017) Potent in vitro synergism of fluconazole and osthole against fluconazole-resistant Candida albicans. Antimicrob Agents Chemother 61:e00436–e00417
Lu H, Zhu ZY, Dong LL, Jia XM, Sun XR, Yan L, Chai YF, Jiang YY, Cao YY (2011) Lack of trehalose accelerates H2O2-induced Candida albicans apoptosis through regulating Ca2+ signaling pathway and caspase activity. PloS One 6:e15808
Lu MJ, Li T, Wan JJ, Li XY, Yuan L, Sun SJ (2017) Antifungal effects of phytocompounds on Candida species alone and in combination with fluconazole. Int J Antimicrob Agents 49:125–136
Lu Q, Jiang MH, Jiang JG, Zhang RF, Zhang MW (2012) Isolation and identification of compounds from Penthorum chinense Pursh with antioxidant and antihepatocarcinoma properties. J Agric Food Chem 60:11097–11103
Luk E, Yang M, Jensen LT, Bourbonnais Y, Culotta VC (2005) Manganese activation of superoxide dismutase 2 in the mitochondria of Saccharomyces cerevisiae. J Biol Chem 280:22715–22720
Ma C, Du F, Yan L, He G, He J, Wang C, Rao G, Jiang Y, Xu G (2015) Potent activities of roemerine against Candida albicans and the underlying mechanisms. Molecules 20:17913–17928
Nobile CJ, Andes DR, Nett JE, Smith FJ, Yue F, Phan QT, Edwards JE, Filler SG, Mitchell AP (2006) Critical role of Bcr1-dependent adhesins in C. albicans biofilm formation in vitro and in vivo. PLoS Pathog 2:e63
Pierce CG, Uppuluri P, Tristan AR, Wormley FL Jr, Mowat E, Ramage G, Lopez-Ribot JL (2008) A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500
Pfaller MA, Castanheira M (2016) Nosocomial candidiasis: antifungal stewardship and the importance of rapid diagnosis. Med Mycol 54:1–22
Prasad R, Shah AH, Rawal MK (2016) Antifungals: mechanism of action and drug resistance. Adv Exp Med Biol 892:327–349
Quan H, Cao YY, Xu Z, Zhao JX, Gao PH, Qin XF, Jiang YY (2006) Potent in vitro synergism of fluconazole and berberine chloride against clinical isolates of Candida albicans resistant to fluconazole. Antimicrob Agents Chemother 50:1096–1099
Ramage G, Vande Walle K, Wickes BL, López-Ribot JL (2001) Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 45:2475–2479
Shen J, Zhang C, Liu Y, Zhao M, Wang Q, Li P, Liu R, Wai Wong VK, Zhang C, Sun X (2023) L-type calcium ion channel-mediated activation of autophagy in vascular smooth muscle cells via thonningianin A (TA) alleviates vascular calcification in type 2 diabetes mellitus. Eur J Pharmacol 15(959):176084
Sokol-Anderson ML, Brajtburg J, Medoff G (1986) Amphotericin B-induced oxidative damage and killing of C. albicans. J Infect Dis 154:76–83
Somer A, Törün SH, Salman N (2011) Caspofungin: the first licensed antifungal drug of the novel echinocandin class. Expert Rev Anti Infect Ther 9:347–355
Sundstrom P (2002) Adhesion in Candida spp. Cell Microbiol 4:461–469
Tyler DD (1975) Polarographic assay and intracellular distribution of superoxide dismutase in rat liver. Biochem J 147:493–504
Wang Y, Cao YY, Jia XM, Cao YB, Gao PH, Fu XP, Ying K, Chen WS, Jiang YY (2006) Cap1p is involved in multiple pathways of oxidative stress response in Candida albicans. Free Radic Biol Med 40:1201–1209
Wang Y (2015) Looking into Candida albicans infection, host response, and antifungal strategies. Virulence 6:307–308
Wiederhold NP (2017) Antifungal resistance: current trends and future strategies to combat. Infect Drug Resist 10:249–259
Yan Y, Tan F, Miao H, Wang H, Cao YY (2019) Effect of Shikonin against Candida albicans biofilms. Front Microbiol 10:1085
Zhao LY, Jiang JC, Zhu ZY, Liao ZB, Yao XW, Yang Y, Cao YY, Jiang YY (2016) Lysine enhances the effect of amphotericin B against Candida albicans in vitro. Acta Biochim Biophys Sin 48:182–193
Zhang TT, Yang L, Jiang JG (2015) Effects of Thonningianin A in natural foods on apoptosis and cell cycle arrest of HepG-2 human hepatocellular carcinoma cells. Food Funct 6:2588–2597
Zhou XG, Qiu WQ, Yu L, Pan R, Teng JF, Sang ZP, Law BY, Zhao Y, Zhang L, Yan L, Tang Y, Sun XL, Wong VKW, Yu CL, Wu JM, Qin DL, Wu AG (2022) Targeting microglial autophagic degradation of the NLRP3 inflammasome for identification of thonningianin A in Alzheimer's disease. Inflamm Regen 42:25
Zhang X, De Micheli M, Coleman ST, Sanglard D, Moye-Rowley WS (2000) Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol Microbiol 36:618–629
Funding
This work was supported by the Natural Science Foundation of China (81671991).
Author information
Authors and Affiliations
Contributions
CYY and ZZY: conceptualization, study design, data analysis, and writing. WH and LH: experimental procedures, data analysis, and writing. LZW: experimental procedures.
Corresponding authors
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Li, H., Liu, Z. et al. Activity of thonningianin A against Candida albicans in vitro and in vivo. Appl Microbiol Biotechnol 108, 96 (2024). https://doi.org/10.1007/s00253-023-12996-1
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00253-023-12996-1