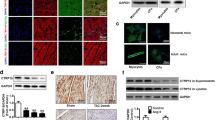
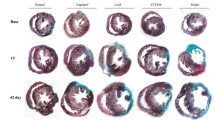
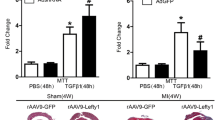
Abstract
Cardiac fibrosis is a remodeling process of the cardiac interstitium, characterized by abnormal metabolism of the extracellular matrix, excessive accumulation of collagen fibers, and scar tissue hyperplasia. Persistent activation and transdifferentiation into myofibroblasts of cardiac fibroblasts promote the progression of fibrosis. Transforming growth factor-β1 (TGF-β1) is a pivotal factor in cardiac fibrosis. Latency-associated peptide (LAP) is essential for activating TGF-β1 and its binding to the receptor. Thus, interference with TGF-β1 and the signaling pathways using LAP may attenuate cardiac fibrosis. Recombinant full-length and truncated LAP were previously constructed, expressed, and purified. Their effects on cardiac fibrosis were investigated in isoproterenol (ISO)-induced cardiac fibroblasts (CFs) and C57BL/6 mice. The study showed that LAP and tLAP inhibited ISO-induced CF activation, inflammation, and fibrosis, improved cardiac function, and alleviated myocardial injury in ISO-induced mice. LAP and tLAP alleviated the histopathological alterations and inhibited the elevated expression of inflammatory and fibrosis-related markers in cardiac tissue. In addition, LAP and tLAP decreased the ISO-induced elevated expression of TGF-β, αvβ3, αvβ5, p-Smad2, and p-Smad3. The study indicated that LAP and tLAP attenuated ISO-induced cardiac fibrosis via suppressing TGF-β/Smad pathway. This study may provide a potential approach to alleviate cardiac fibrosis.
Key points
• LAP and tLAP inhibited ISO-induced CF activation, inflammation, and fibrosis.
• LAP and tLAP improved cardiac function and alleviated myocardial injury, inflammation, and fibrosis in ISO-induced mice.
• LAP and tLAP attenuated cardiac fibrosis via suppressing TGF-β/Smad pathway.






Similar content being viewed by others
Data availability
The datasets generated during the study are available from the corresponding author on reasonable request.
References
Bishop JE, Laurent GJ (1995) Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J 16 Suppl C:38–44. https://doi.org/10.1093/eurheartj/16.suppl_c.38
Camelliti P, Borg TK, Kohl P (2005) Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res 65(1):40–51. https://doi.org/10.1016/j.cardiores.2004.08.020
Dong RQ, Wang ZF, Zhao C, Gu HR, Hu ZW, Xie J, Wu YQ (2015) Toll-like receptor 4 knockout protects against isoproterenol-induced cardiac fibrosis: the role of autophagy. J Cardiovasc Pharmacol Ther 20(1):84–92. https://doi.org/10.1177/1074248414539564
Henderson NC, Sheppard D (2013) Integrin-mediated regulation of TGFbeta in fibrosis. Biochim Biophys Acta 1832(7):891–896. https://doi.org/10.1016/j.bbadis.2012.10.005
Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY (2018) New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact 292:76–83. https://doi.org/10.1016/j.cbi.2018.07.008
Jiang XH, Wu QQ, Xiao Y, Yuan Y, Yang Z, Bian ZY, Chang W, Tang QZ (2017) Evodiamine prevents isoproterenol-induced cardiac fibrosis by regulating endothelial-to-mesenchymal transition. Planta Med 83(9):761–769. https://doi.org/10.1055/s-0042-124044
Khalil N (1999) TGF-beta: from latent to active. Microbes Infect 1(15):1255–1263. https://doi.org/10.1016/s1286-4579(99)00259-2
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell Mol Life Sci: CMLS 71(4):549–574. https://doi.org/10.1007/s00018-013-1349-6
Krenek P, Kmecova J, Kucerova D, Bajuszova Z, Musil P, Gazova A, Ochodnicky P, Klimas J, Kyselovic J (2009) Isoproterenol-induced heart failure in the rat is associated with nitric oxide-dependent functional alterations of cardiac function. Eur J Heart Fail 11(2):140–146. https://doi.org/10.1093/eurjhf/hfn026
Li AH, Liu PP, Villarreal FJ, Garcia RA (2014) Dynamic changes in myocardial matrix and relevance to disease: translational perspectives. Circ Res 114(5):916–927. https://doi.org/10.1161/CIRCRESAHA.114.302819
Li N, Zhou H, Ma ZG, Zhu JX, Liu C, Song P, Kong CY, Wu HM, Deng W, Tang QZ (2018) Geniposide alleviates isoproterenol-induced cardiac fibrosis partially via SIRT1 activation in vivo and in vitro. Front Pharmacol 9:854. https://doi.org/10.3389/fphar.2018.00854
Li Z (2021) New insights into the pathogenesis of systemic mastocytosis. Int J Mol Sci 22(9). https://doi.org/10.3390/ijms22094900
Liu M, de Juan L, Abad B, Cheng K (2021) Cardiac fibrosis: myofibroblast-mediated pathological regulation and drug delivery strategies. Adv Drug Deliv Rev 173:504–519. https://doi.org/10.1016/j.addr.2021.03.021
Ludbrook SB, Barry ST, Delves CJ, Horgan CM (2003) The integrin alphavbeta3 is a receptor for the latency-associated peptides of transforming growth factors beta1 and beta3. Biochem J 369(Pt 2):311–318. https://doi.org/10.1042/BJ20020809
Nuamnaichati N, Sato VH, Moongkarndi P, Parichatikanond W, Mangmool S (2018) Sustained beta-AR stimulation induces synthesis and secretion of growth factors in cardiac myocytes that affect on cardiac fibroblast activation. Life Sci 193:257–269. https://doi.org/10.1016/j.lfs.2017.10.034
Robertson IB, Horiguchi M, Zilberberg L, Dabovic B, Hadjiolova K, Rifkin DB (2015) Latent TGF-beta-binding proteins. Matrix Biol 47:44–53. https://doi.org/10.1016/j.matbio.2015.05.005
Saadat S, Noureddini M, Mahjoubin-Tehran M, Nazemi S, Shojaie L, Aschner M, Maleki B, Abbasi-Kolli M, RajabiMoghadam H, Alani B, Mirzaei H (2020) Pivotal role of TGF-beta/Smad signaling in cardiac fibrosis: non-coding RNAs as effectual players. Front Cardiovasc Med 7:588347. https://doi.org/10.3389/fcvm.2020.588347
Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B (2014) Integrins alphavbeta5 and alphavbeta3 promote latent TGF-beta1 activation by human cardiac fibroblast contraction. Cardiovasc Res 102(3):407–417. https://doi.org/10.1093/cvr/cvu053
Song HF, He S, Li SH, Wu J, Yin W, Shao Z, Du GQ, Wu J, Li J, Weisel RD, Verma S, Xie J, Li RK (2020) Knock-out of MicroRNA 145 impairs cardiac fibroblast function and wound healing post-myocardial infarction. J Cell Mol Med 24(16):9409–9419. https://doi.org/10.1111/jcmm.15597
Song X, Qiu Y, Shi J, Li L, Yuan X, Wu D, Chu Y (2022a) Prokaryotic expression, purification and evaluation of anti-cardiac fibrosis activity of recombinant TGF-beta latency associated peptide. PeerJ 10:e12797. https://doi.org/10.7717/peerj.12797
Song X, Shi J, Liu J, Liu Y, Yu Y, Qiu Y, Cao Z, Pan Y, Yuan X, Chu Y, Wu D (2022b) Recombinant truncated latency-associated peptide alleviates liver fibrosis in vitro and in vivo via inhibition of TGF-beta/Smad pathway. Mol Med 28(1):80. https://doi.org/10.1186/s10020-022-00508-2
Su C, Wang Q, Luo H, Jiao W, Tang J, Li L, Tian L, Chen X, Liu B, Yu X, Li S, Guo S, Wang W (2020) Si-Miao-Yong-An decoction attenuates cardiac fibrosis via suppressing TGF-beta1 pathway and interfering with MMP-TIMPs expression. Biomed Pharmacother 127:110132. https://doi.org/10.1016/j.biopha.2020.110132
Sun M, Yu H, Zhang Y, Li Z, Gao W (2015) MicroRNA-214 mediates isoproterenol-induced proliferation and collagen synthesis in cardiac fibroblasts. Sci Rep 5:18351. https://doi.org/10.1038/srep18351
Tzavlaki K, Moustakas A (2020) TGF-beta signaling. Biomolecules 10(3) https://doi.org/10.3390/biom10030487
van Nieuwenhoven FA, Turner NA (2013) The role of cardiac fibroblasts in the transition from inflammation to fibrosis following myocardial infarction. Vascul Pharmacol 58(3):182–188. https://doi.org/10.1016/j.vph.2012.07.003
Weber KT, Sun Y, Bhattacharya SK, Ahokas RA, Gerling IC (2013) Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol 10(1):15–26. https://doi.org/10.1038/nrcardio.2012.158
Wei Y, Wu Y, Feng K, Zhao Y, Tao R, Xu H, Tang Y (2020) Astragaloside IV inhibits cardiac fibrosis via miR-135a-TRPM7-TGF-beta/Smads pathway. J Ethnopharmacol 249:112404. https://doi.org/10.1016/j.jep.2019.112404
Yoshida K, Matsuzaki K, Murata M, Yamaguchi T, Suwa K, Okazaki K (2018) Clinico-pathological importance of TGF-beta/phospho-Smad signaling during human hepatic fibrocarcinogenesis. Cancers 10(6). https://doi.org/10.3390/cancers10060183
Funding
This study was supported by the Natural Science Foundation of Heilongjiang Province (Outstanding Youth Project, Grant No. YQ2020H026), Central Finance supports Local Colleges, and Universities Talent Development Funding from Heilongjiang Provincial Department of Finance (High Level Talent Support Project, Grant No. 2020GSP09), Torch Program Youth Top Talent Project of Mudanjiang Medical University (Grant No. 2022-MYHJ-015), Doctoral Research Foundation of Mudanjiang Medical University (Grant No. 2021-MYBSKY-066), and Scientific research project of Health Commission of Heilongjiang Province (Grant No. 20220101040974).
Author information
Authors and Affiliations
Contributions
DW and XHY conceived and designed research. YFQ, XDS, ZQC, KKZ, and JRL conducted experiments. YW, YL, and YHC contributed new reagents or analytical tools. JYS, FZ, and YP analyzed data. YFQ and XDS wrote the manuscript. DW revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the Institutional Animal Care and Use Committee of Mudanjiang Medical University (IACUC-20210702–9).
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, Y., Song, X., Liu, Y. et al. Application of recombinant TGF-β1 inhibitory peptide to alleviate isoproterenol-induced cardiac fibrosis. Appl Microbiol Biotechnol 107, 6251–6262 (2023). https://doi.org/10.1007/s00253-023-12722-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12722-x