Abstract
The p30 protein is abundantly expressed in the early stage of African swine fever virus (ASFV) infection. Thus, it is an ideal antigen candidate for serodiagnosis with the use of an immunoassay. In this study, a chemiluminescent magnetic microparticle immunoassay (CMIA) was developed for the detection of antibodies (Abs) against ASFV p30 protein in porcine serum. Purified p30 protein was coupled to magnetic beads, and the experimental conditions including concentration, temperature, incubation time, dilution ratio, buffers, and other relevant variables were evaluated and optimized. To evaluate the performance of the assay, a total of 178 pig serum samples (117 negative and 61 positive samples) were tested. According to receiver operator characteristic curve analysis, the cut-off value of the CMIA was 104,315 (area under the curve, 0.998; Youden’s index, 0.974; 95% confidence interval: 99.45 to 100%). Sensitivity results showed that the dilution ratio of p30 Abs in ASFV-positive sera detected by the CMIA is much higher when compared to commercial blocking ELISA kit. Specificity testing showed that no cross-reactivity was observed with sera positive for other porcine disease viruses. The intraassay coefficient of variation (CV) was < 5%, and the interassay CV was < 10%. The p30-magnetic beads could be stored at 4 °C for more than 15 months without loss of activity. The kappa coefficient between CMIA and INGENASA blocking ELISA kit was 0.946, showing strong agreement. In conclusion, our method showed superiority with high sensitivity, specificity, reproducibility, and stability and potentialized its application in the development of a diagnostic kit for the detection of ASF in clinical samples.
Key points
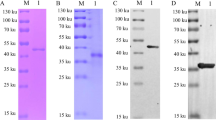
• ASFV tag-free p30 was successfully purified.
• High sensitivity, specificity, relatively simple, and time-saving to detect antibody against ASFV were developed.
• The development of CMIA will help the clinical diagnosis of ASFV and will be useful for large-scale serological test.







Similar content being viewed by others
Data availability
The data that support the results of this study are available from the corresponding author upon reasonable request.
References
Afonso CL, Alcaraz C, Brun A, Sussman MD, Onisk DV, Escribano JM, Rock DL (1992) Characterization of P30, a highly antigenic membrane and secreted protein of African Swine Fever Virus. Virology 189(1):368–373. https://doi.org/10.1016/0042-6822(92)90718-5
Barderas MG, Rodriguez F, Gomez-Puertas P, Aviles M, Beitia F, Alonso C, Escribano JM (2001) Antigenic and immunogenic properties of a chimera of two immunodominant African swine fever virus proteins. Arch Virol 146(9):1681–1691. https://doi.org/10.1007/s007050170056
Bergeron HC, Glas PS, Schumann KR (2017) Diagnostic specificity of the African swine fever virus antibody detection enzyme-linked immunosorbent assay in feral and domestic pigs in the United States. Transbound Emerg Dis 64(6):1665–1668. https://doi.org/10.1111/tbed.12717
Blome S, Gabriel C, Beer M (2013) Pathogenesis of African swine fever in domestic pigs and European wild boar. Virus Res 173(1):122–130. https://doi.org/10.1016/j.virusres.2012.10.026
Borca MV, Ramirez-Medina E, Silva E, Vuono E, Rai A, Pruitt S, Holinka LG, Velazquez-Salinas L, Zhu J, Gladue DP (2020) Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene results in sterile immunity against the current epidemic Eurasia strain. J Virol 94(7):e02017–e02019. https://doi.org/10.1128/JVI.02017-19
Chen C, Guo X, Liang H, Ning B, Li J, Zhong S, Liu X, Li L (2019) Determination of parvovirus antibodies in canine serum using magnetic bead-based chemiluminescence immunoassay. Biotechnol Appl Biochem 66(4):586–590. https://doi.org/10.1002/bab.1758
Chen W, Zhao D, He X, Liu R, Wang Z, Zhang X, Li F, Shan D, Chen H, Zhang J, Wang L, Wen Z, Wang X, Guan Y, Liu J, Bu Z (2020) A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci China Life Sci 63(1674-7305):623. https://doi.org/10.1007/s11427-020-1657-9
Chen X, Chen X, Liang Y, Xu S, Weng Z, Gao Q, Huang Z, Zhang G, Gong L (2022) Interaction network of African swine fever virus structural protein p30 with host proteins. Front Microbiol 13:971888. https://doi.org/10.3389/fmicb.2022.971888
Cinquanta L, Fontana DE, Bizzaro N (2017) Chemiluminescent immunoassay technology: what does it change in autoantibody detection? Autoimmun Highlights 8(1):1–8. https://doi.org/10.1007/s13317-017-0097-2
Cubillos C, Gómez-Sebastian S, Moreno N, Nuñez MC, Mulumba-Mfumu LK, Quembo CJ, Heath L, Etter EM, Jori F, Escribano JM, Blanco E (2013) African swine fever virus serodiagnosis: a general review with a focus on the analyses of African serum samples. Virus Res 173(1):159–167. https://doi.org/10.1016/j.virusres.2012.10.021
Cui C, Liu P, Feng Z, Xin R, Yan C, Li Z (2015) Evaluation of the clinical effectiveness of HIV antigen/antibody screening using a chemiluminescence microparticle immunoassay. J Virol Methods 214:33–36. https://doi.org/10.1016/j.jviromet.2014.07.026
Dixon LK, Chapman DAG, Netherton CL, Upton C (2013) African swine fever virus replication and genomics. Virus Res 173(1):3–14. https://doi.org/10.1016/j.virusres.2012.10.020
Forth JH, Forth LF, Lycett S, Bell-Sakyi L, Keil GM, Blome S, Calvignac-Spencer S, Wissgott A, Krause J, Hoper D, Kampen H, Beer M (2020) Identification of African swine fever virus-like elements in the soft tick genome provides insights into the virus’ evolution. BMC Biol 18(1):1–18. https://doi.org/10.1186/s12915-020-00865-6
Gimenez-Lirola LG, Mur L, Rivera B, Mogler M, Sun Y, Lizano S, Goodell C, Harris DL, Rowland RR, Gallardo C, Sanchez-Vizcaino JM, Zimmerman J (2016) Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PloS One 11(9):e0161230. https://doi.org/10.1371/journal.pone.0161230
Jia N, Ou Y, Pejsak Z, Zhang Y, Zhang J (2017) Roles of African swine fever virus structural proteins in viral infection. J Vet Res 61(2):135–143. https://doi.org/10.1515/jvetres-2017-0017
Jin H, Lin JM, Wang X, Xin TB, Liang SX, Li ZJ, Hu GM (2009) Magnetic particle-based chemiluminescence enzyme immunoassay for free thyroxine in human serum. J Pharm Biomed Anal 50(5):891–896. https://doi.org/10.1016/j.jpba.2009.06.011
Kazakova AS, Imatdinov IR, Dubrovskaya OA, Imatdinov AR, Sidlik MV, Balyshev VM, Krasochko PA, Sereda AD (2017) Recombinant protein p30 for serological diagnosis of African swine fever by immunoblotting assay. Transbound Emerg Dis 64(5):1479–1492. https://doi.org/10.1111/tbed.12539
Leary TP, Gutierrez RA, Muerhoff AS, Birkenmeyer LG, Desai SM, Dawson GJ (2006) A chemiluminescent, magnetic particle-based immunoassay for the detection of hepatitis C virus core antigen in human serum or plasma. J Med Virol 78(11):1436–1440. https://doi.org/10.1002/jmv.20716
Liu L, Luo Y, Accensi F, Ganges L, Rodríguez F, Shan H, Ståhl K, Qiu HJ, Belák S (2017) Pre-clinical evaluation of a real-time PCR assay on a portable instrument as a possible field diagnostic tool: experiences from the testing of clinical samples for african and classical swine fever viruses. Transbound Emerg Dis 64(5):e31–e35. https://doi.org/10.1111/tbed.12538
Liu YN, Xie ZH, Li Y, Song YY, Di DD, Liu JY, Gong L, Chen ZY, Wu JX, Ye ZQ, Liu JQ, Yu WQ, Lv L, Zhong QP, Tian CW, Song QQ, Wang H, Chen HJ (2023) Evaluation of an I177L gene-based five-gene-deleted African swine fever virus as a live attenuated vaccine in pigs. Emerg Microbes Infec 12(1):2148560
Luo Y, Atim SA, Shao L, Ayebazibwe C, Sun Y, Liu Y, Ji S, Meng XY, Li S, Li Y, Masembe C, Ståhl K, Widén F, Liu L, Qiu HJ (2017) Development of an updated PCR assay for detection of African swine fever virus. Arch Virol 162(1):191–199. https://doi.org/10.1007/s00705-016-3069-3
Neilan JG, Zsak L, Lu Z, Burrage TG, Kutish GF, Rock DL (2004) Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 319(2):337–342. https://doi.org/10.1016/j.virol.2003.11.011
Oh T, Nguyen TM, Ngo TTN, Thinh D, Nguyen TTP, Do LD, Do DT (2021) Long-term follow-up of convalescent pigs and their offspring after an outbreak of acute African swine fever in Vietnam. Transbound Emerg Dis 68(6):3194–3199. https://doi.org/10.1111/tbed.14276
Oviedo JM, Rodriguez F, Gomez-Puertas P, Brun A, Gomez N, Alonso C, Escribano JM (1997) High level expression of the major antigenic African swine fever virus proteins p54 and p30 in baculovirus and their potential use as diagnostic reagents. J Virol Methods 64(1):27–35. https://doi.org/10.1016/s0166-0934(96)02140-4
Pan IC, Huang TS, Hess WR (1982) New method of antibody detection by indirect immunoperoxidase plaque staining for serodiagnosis of African swine fever. J Clin Microbiol 16(4):650–655. https://doi.org/10.1128/jcm.16.4.650-655.1982
Pastor MJ, Laviada MD, Sanchez-Vizcaino JM, Escribano JM (1989) Detection of African swine fever virus antibodies by immunoblotting assay. Can J Vet Res 53(1):105–107. https://doi.org/10.1007/978-3-7091-3987-5_1
Simoes M, Freitas FB, Leitao A, Martins C, Ferreira F (2019) African swine fever virus replication events and cell nucleus: new insights and perspectives. Virus Res 270:197667. https://doi.org/10.1016/j.virusres.2019.197667
Sun E, Huang L, Zhang X, Zhang J, Shen D, Zhang Z, Wang Z, Huo H, Wang W, Huangfu H, Wang W, Li F, Liu R, Sun J, Tian Z, Xia W, Guan Y, He X, Zhu Y et al (2021a) Genotype I African swine fever viruses emerged in domestic pigs in China and caused chronic infection. Emerg Microbes Infect 10(1):2183–2193. https://doi.org/10.1080/22221751.2021.1999779
Sun E, Zhang Z, Wang Z, He X, Zhang X, Wang L, Wang W, Huang L, Xi F, Huangfu H, Tsegay G, Huo H, Sun J, Tian Z, Xia W, Yu X, Li F, Liu R, Guan Y et al (2021b) Emergence and prevalence of naturally occurring lower virulent African swine fever viruses in domestic pigs in China in 2020. Sci China Life Sci 64(5):752–765. https://doi.org/10.1007/s11427-021-1904-4
Vidal MI, Stiene M, Henkel J, Bilitewski U, Costa JV, Oliva AG (1997) A solid-phase enzyme linked immunosorbent assay using monoclonal antibodies, for the detection of African swine fever virus antigens and antibodies. J Virol Methods 66(2):211–218. https://doi.org/10.1016/s0166-0934(97)00059-1
Wang T, Sun Y, Qiu H-J (2018) African swine fever: an unprecedented disaster and challenge to China. Infect Dis Poverty 7(1):111. https://doi.org/10.1186/s40249-018-0495-3
Xin TB, Liang SX, Wang X, Li H, Lin JM (2008) Determination of estradiol in human serum using magnetic particles-based chemiluminescence immunoassay. Anal Chim Acta 627(2):277–284. https://doi.org/10.1016/j.aca.2008.08.020
Zhang GL, Liu W, Gao Z, Yang SC, Zhou GQ, Chang YY, Ma YY, Liang XX, Shao JJ, Chang HY (2021) Antigenicity and immunogenicity of recombinant proteins comprising African swine fever virus proteins p30 and p54 fused to a cell-penetrating peptide. Int Immunopharmacol 101:108251. https://doi.org/10.1016/j.intimp.2021.108251
Zhang QY, Chen H, Lin Z, Lin JM (2011) Chemiluminescence enzyme immunoassay based on magnetic nanoparticles for detection of hepatocellular carcinoma marker glypican-3. J Pharm Anal 1(3):166–174. https://doi.org/10.1016/j.jpha.2011.06.004
Funding
This work was supported by the National Key R&D Program of China (2021YFD1801300), Gansu Province Animal Biological Products Innovation Consortium Project (22ZD6NA012), the earmarked fund for CARS-35 (CARS-35), open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province (2022SDZG02).
Author information
Authors and Affiliations
Contributions
Y. H., H. T., and H. Z. designed the study. Z. S., L. C., J. L., and Q. Z. performed the experiments. G. Z. contributed new reagents. X. L. provided the serum samples. Z. S. and L. C. analyzed the data. L. C. wrote the manuscript. All authors discussed the results.
Corresponding authors
Ethics declarations
Ethical approval
Experiments involving animals were approved by Lanzhou Veterinary Research Institute.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, Z., Cao, L., Luo, J. et al. A chemiluminescent magnetic microparticle immunoassay for the detection of antibody against African swine fever virus. Appl Microbiol Biotechnol 107, 3779–3788 (2023). https://doi.org/10.1007/s00253-023-12518-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12518-z