Abstract
Yeast cells face various stress factors during industrial fermentations, since they are exposed to harsh environmental conditions, which may impair biomolecules productivity and yield. In this work, the use of an antioxidant peptide extract obtained from industrial spent yeast was explored as supplement for Saccharomyces cerevisiae fermentation to prevent a common bottleneck: oxidative stress. For that, a recombinant yeast strain, producer of β-farnesene, was firstly incubated with 0.5 and 0.7 g/L peptide extract, in the presence and absence of hydrogen peroxide (an oxidative stress inducer), for 1–5 h, and then assayed for intracellular reactive oxygen species, and growth ability in agar spot assays. Results showed that under 2 mM H2O2, the peptide extract could improve cells growth and reduce reactive oxygen species production. Therefore, this antioxidant effect was further evaluated in shake-flasks and 2-L bioreactor batch fermentations. Peptide extract (0.7 g/L) was able to increase yeast resistance to the oxidative stress promoted by 2 mM H2O2, by reducing reactive oxygen species levels between 1.2- and 1.7-fold in bioreactor and between 1.2- and 3-fold in shake-flask fermentations. Moreover, improvements on yeast cell density of up to 1.5-fold and 2-fold, and on biomolecule concentration of up to 1.6-fold and 2.8-fold, in bioreactor and shake-flasks, respectively, were obtained. Thus, culture medium supplementation with antioxidant peptide extracted from industrial spent yeast is a promising strategy to improve fermentation performance while valuing biomass waste. This valorization can promote a sustainable and eco-friendly solution for the biotechnology industry by the implementation of a circular economy model.
Key points
• Peptide extract from spent yeast applied for the first time on yeast fermentation.
• Antioxidant peptide extract enhanced S. cerevisiae oxidative stress resistance.
• Fermentation performance under stress improved by peptide extract supplementation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Industrial biotechnology sights the production of chemicals, proteins, metabolites, biofuels, and other products in a sustainable manner. Saccharomyces cerevisiae is widely used and studied in industrial fermentation due its fast growth rate, strong ability to adapt to large scale fermentation, high tolerance against harsh conditions, and relatively easy genetic manipulation (Carsanba et al. 2021; Nandy and Srivastava 2018; Stalidzans and Dace 2021). Microbial fermentation allows the production of natural molecules with high yield, low environmental impact, and without exploring natural resources (Nandy and Srivastava 2018). Despite these numerous advantages, industrial fermentation also produce waste. One of the major wastes is spent yeast biomass, which also contains compounds with commercial interest, due to their several biological activities (antihypertensive, antioxidant, and antimicrobial activities) (Oliveira et al. 2022a; San Martin et al. 2021). Thus, spent yeast is a valuable fermentation residue for extracting biologically active compounds for distinct applications, including dietary supplements and cosmetics (Oliveira et al. 2022a).
During industrial fermentations, yeast cells face several environmental stresses such as heat, osmotic, oxidative, pH, and ethanol, that can impair their fermentation efficiency (Deparis et al. 2017; Saini et al. 2018). Some of these harsh conditions can trigger the accumulation of intracellular reactive oxygen species (ROS), which promote yeast oxidative stress (Saini et al. 2018; Walker and Basso 2020). Nevertheless, ROS are free radicals generated also by intracellular metabolism processes, like aerobic respiration in mitochondria. Oxidative stress can lead to protein oxidation, lipid peroxidation, DNA damage, and thus triggering programmed cell death (apoptosis) (Deparis et al. 2017; Saini et al. 2018).
Under normal physiological conditions, ROS are neutralized by the activation of enzymatic and non-enzymatic defence mechanisms to maintain the cellular redox balance and to resist oxidative stress (Auesukaree 2017; Deparis et al. 2017; Oliveira et al. 2022a). The antioxidant enzymatic defence system consists of a set of protective enzymes (superoxide dismutases (SODs), catalases (CATs), glutathione peroxidases (GPXs), and peroxiredoxins (Prxs)) which can be upregulated to detoxify ROS and maintain the intracellular redox environment in a reduced state (Auesukaree 2017; Deparis et al. 2017; Eigenfeld et al. 2021). These enzymes develop a complex and tight detoxification process that starts when the SODs convert superoxide anion to hydrogen peroxide (H2O2), which is then reduced to water and oxygen (Auesukaree 2017; San Martin et al. 2021). The non-enzymatic defence mechanisms correspond to small molecules, such as glutathione, D-erythroascorbate, trehalose, and ubiquinol, which act as radical scavengers (Auesukaree 2017).
However, when ROS levels are too high, the endogenous defence mechanisms are not enough to protect the cells from oxidative damages. The most described methods to prevent oxidative damage are medium optimization and yeast engineering. The first one is through the addition of known antioxidants, such as phenolic and carotenoid compounds into culture medium (Carvalho et al. 2022; Mendes et al. 2015; Qiu et al. 2019). The supplementation of medium with antioxidant divalent metal ions (such as Zn2+, Ca2+, and Mg2+) and amino acids (such as arginine, proline, tyrosine, and tryptophan) is also a common strategy to improve yeast stress tolerance (Qiu et al. 2019; Takagi 2021). On the other hand, the overexpression of proteins and enzymes with antioxidant capacity, like CAT, SOD, and GSH, or deleting genes such as proline dehydrogenase (Put1) can also lead to reduced cell stress (Li et al. 2020; Qiu et al. 2019).
A possible and unexplored application of the antioxidant compounds extracted from spent yeast would be their use in fermentation as an antioxidant supplement to reduce yeast oxidative stress. Reusing spent yeast to extract bioactive compounds allows the valorization of fermentation waste and promotes a circular economy policy (Faustino et al. 2021). Thus, the application of these extracts in yeast fermentation may improve the profitability of the fermentation process, by both bio-valorizing fermentation waste and improving yeast performance in a circular economy approach. According to that, this work aims to study the capacity of an antioxidant peptide-rich extract (APE), isolated from Amyris Inc., Emeryville, CA, USA, spent yeast (Oliveira et al. 2022b), to reduce oxidative stress in S. cerevisiae fermentation. In order to accomplish that, a genetically modified yeast producer of β-farnesene was exposed to 2 mM H2O2 (a stress inducer), in the presence and absence of APE, during shake-flasks and bioreactor fermentations. β-farnesene is currently produced by yeast fermentation at industrial scale (200,000 m3) (Benjamin et al. 2016; Meadows et al. 2016), in which the yeast faces elevated oxidative stress. As such, a recombinant yeast producer of β-farnesene, precursor of the yeast strains that have been used for farnesene manufacturing in recent years by Amyris Inc., was chosen as a model for this work. Besides the variations on the intracellular ROS levels, the antioxidant effect of APE on yeast growth and biomolecule production was also evaluated.
Materials and methods
Strain and culture media
The strain used in this study was a S. cerevisiae (strain: CEN.PK2, BioSample: SAMN27162618) engineered by Amyris Inc., Emeryville, USA, to produce β-farnesene constitutively. A pre-culture of this strain was grown in 63 mL of culture medium in one of the 250-mL Erlenmeyer flasks (with four baffles), inoculated with two cryovial (containing 1 mL of culture volume each), and incubated at 30 °C and 200 rpm in an orbital incubator for 42 h. The standard medium culture contained yeast extract (5 g/L), succinic acid buffer (6 g/L), potassium phosphate monobasic (1 g/L), magnesium sulfate heptahydrate (0.5 g/L), sucrose (60 g/L), and trace metals and vitamins as described by Hoek et al. (2000). Agar plates contained tryptophan (0.02 g/L), yeast nitrogen base without amino acids (6.7 g/L), yeast synthetic drop-out medium supplements (2 g/L), sucrose (2 g/L), maltose (1 g/L), and lysine (2 g/L). All reagents used in this study were from Sigma Aldrich, (St. Louis, MO, USA). APE was produced from spent yeast waste, resulting of Amyris Inc., Emeryville, USA, fermentations, by selective membrane filtration (Oliveira et al. 2022b).
APE extraction
Peptide-rich extracts were obtained from waste streams of mannan (Mpep) extraction from spent yeast (S. cerevisiae) as described in the paper by Oliveira et al. (2022b). APE corresponded to the Mpep > 1 kDa and contained 86.4 ± 8.7% (w/w) protein, 7.44 ± 0.22% (w/w) of sugars and minerals (5.22 ± 0.01 ng/g extract of potassium, 1.04 ± 0.01 ng/g extract of magnesium, and 0.10 ± 0.01 ng/g extract of calcium).
Antioxidant activity
Antioxidant activity of APE was determined by 2,2′-azinobis(3-ethyl-benzothiazoline-6-sulphonate)-radical (ABTS +) scavenging activity as described by Oliveira et al. (2022b).
Spot assay
First, an optimization of oxidative stress induction in S. cerevisiae was performed for different H2O2 concentrations (1, 2, 5, and 10 mM), and times of exposure, according to the literature (Khan et al. 2005; Pedreño et al. 2002; Coelho et al. 2019). The following experiments were conducted using 2 and 5 mM H2O2 (with one and 5 h of incubation) since these were the conditions presenting the best impact on yeast membrane integrity and ROS production. Furthermore, the concentrations of APE selected were based on its water solubility, which was ≤ 0.7 g/L.
Pre-culture was harvested and suspended in 3 mL of liquid culture medium in 15 mL Falcons to an initial washed optical density at 600 nm (wOD600) of 1.0. In the control Falcon, cells were cultivated using standard culture medium, while in the other Falcons, cells were suspended in medium containing 2 mM or 5 mM H2O2 with and without the addition of 0.5 or 0.7 g/L APE and in medium containing 2 mM or 5 mM H2O2 with and without the addition of 0.5 and 1 mM of ascorbic acid (AA) (Fig. 1). Prepared Falcons were incubated for 5 h at 30 °C and 650 rpm on a thermomixer.
Schematic representation of spot assays after 2 and 5 h of exposure to hydrogen peroxide (H2O2) in the presence and absence of ascorbic acid (AA) or antioxidant peptide extract (APE) in 15 mL Falcons, small-scale fermentations (in 1 L shake-flasks) and bioreactor fermentations (in bioreactors with 2.7 L of working volume)
After 1 and 5 h of H2O2 exposure, cells were harvested by centrifugation at 13,500 rpm for 5 min, diluted in sterile 0.9% NaCl to wOD600 of 1.0, and then serially tenfold diluted. One aliquot (4 μL) of each dilution from each sample was transferred to agar plates and incubated at 30 °C for 4 days.
Small scale (shake-flask) fermentation
Pre-culture cells were harvested and suspended in 100 mL of culture medium in several 1 L Erlenmeyer flasks (with 4 baffles) to an initial wOD600 of 0.5. The conditions tested in shake-flasks were standard culture medium (control), medium with 2 mM H2O2, medium with 0.7 g/L of APE (higher concentration possible due to water solubility), and medium with 0.7 g/L of APE plus 2 mM H2O2 (Fig. 1). APE was dissolved in deionized water, mixed with culture medium, and sterilized by filtration. Shake-flasks were incubated in an orbital incubator at 30 °C and 200 rpm until the sugars in medium were consumed. Samples were collected in each 3 h of a working day to determine washed optical density, farnesene concentration, and sugar consumption. Membrane integrity and ROS levels were evaluated in the first sample of each day. Two independent replicates were performed for shake-flasks fermentations. The execution of a third replicate was replaced by the scale-up to bioreactor fermentations to validate the positive effect of APE at a larger scale.
Bioreactor batch fermentation
In order to obtain the inoculum for the batch bioreactor fermentations, two seed flask passages (incubation at 30 °C and 200 rpm) were performed. Incubation times in the first and second seed flask steps were 42 h and 22 h, respectively.
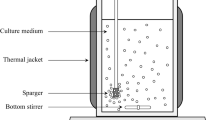
The batch fermentations were performed in reactors (Eppendorf DASGIP parallel bioreactor system, Hamburg, Germany) with 2.7 L of working volume maintaining temperature at 30 °C, pH at 5.0 (adjusted with ammonium hydroxide at 12.5%), aeration at 0.5 L/min, and dissolved oxygen (DO) of at least 30% (controlled by agitation ranging between 300 and 1200 rpm). Batch reactors had an initial volume of 1-L after inoculation (10% inoculum, initial wOD600 of ~ 1). Cells were cultured until all sugars in medium were consumed (~ 47 h). Different conditions were tested: reactor 1 contained the standard medium (control), reactor 2 medium with 2 mM H2O2, reactor 3 medium with 0.7 g/L APE, and reactor 4 medium with 0.7 g/L APE plus 2 mM H2O2 (Fig. 1).
Temperature (°C), pH, DO, agitation, aeration, and off-gas data, such as CO2, O2, oxygen uptake rate (OUR) and ethanol, were monitored and recorded automatically. Whole cell broth samples were collected and prepared for the measurements of farnesene concentration (g/kg), washed optical density, sucrose (g/L), ROS, and cell viability (%).
Washed optical density
Yeast growth was analyzed by the measurement of the wOD600 at the defined sampling points. Samples were centrifuged at 13,500 rpm for 5 min (1 mL), and after discarding the supernatant, the cells were suspended in 1 mL of deionized water and diluted to the absorbance read on the spectrophotometer (Shimadzu UV-1900 UV–VIS Spectrophotometer, Kyoto, Japan) at 600 nm.
Sucrose quantification (g/L)
Sucrose concentrations were determined by high-performance liquid chromatography (HPLC). The supernatant collected during wOD analysis was diluted fivefold in deionized water, filtered, and analyzed in HPLC using the Aminex HPX-87H column (300 × 7.8 mm) with a pre-column (30 × 4.6 mm) and a refractive index detector (RID) (BioRad, Hercules, CA, USA). The column and detector temperatures were set at 50 °C and 35 °C, respectively. Mobile phase of 5 mM of H2SO4 was eluted at 0.6 mL min−1. Six standards with 0.3, 0.6, 1.25, 2.5, 5, and 10 g/L of sucrose were used for the calibration curve determination. Data acquisition was performed using Agilent OpenLab CDS A.01.12.165 (Agilent, Santa Clara, CA, USA).
Membrane integrity
Cellular membrane integrity was determined using the fluorescent probe propidium iodide (IP, Sigma-Aldrich, St. Louis, MO, USA, reference P4864). Cells were incubated with 10 µM propidium iodide at 30 °C for 20 min, and then harvested and suspended in 0.9% NaCl (w/v). The cells used as positive controls were treated with 70% ethanol for 10 min prior to incubation with IP. Fluorescence intensity was measured by flow cytometry (BD Accuri™ C6 Plus Personal Flow Cytometer; Accuri, Ann Arbor, MI, USA) using the FL-2 channel (585/40 nm) and reading 20 000 events.
Measurement of ROS
The oxidant-sensitive fluorescent probe dihydrorhodamine (DHR; Sigma-Aldrich, St. Louis, MO, USA) was used to determine the intracellular ROS levels. Cells were treated with 50 µM DHR in culture media for 1 h, and then washed with 0.9% NaCl twice and suspended in 200 µL of 0.9% NaCl. Cells treated with 20 µM carbonyl cyanide 3-chlorophenylhydrazone (CCCP) during 10 min before incubation with DHR were used as positive control. Fluorescence was measured in FL-1 channel (533/530 nm) in a BD Accuri™ C6 plus Personal Flow Cytometer. Data was acquired from a total of 20,000 events/samples from 2 independent assays. Quantification of intracellular ROS was expressed by relative fluorescence units (RFU) calculated in relation to sample control (unlabeled cells).
Farnesene determination
Farnesene determination was performed by gas chromatography (GC), using an Agilent 8890 GC System with a flame ionization detector (FID) (Agilent, Santa Clara, CA, USA. Two different standard farnesene solutions (low and high concentrations) and the samples were injected after performing a farnesene extraction protocol. Briefly, farnesene extraction was applied by mixing fermentation broth with a solution containing 10% 2-butoxyethanol, 0.25% tetradecane, and 90% methanol at a proportion of 1:20 or 1:40 (shake-flask or reactor samples, respectively). Samples were injected in a separation column (Phenomenex ZB-5HT Inferno; Phenomenex, Torrance, CA, USA, with 30 + 5 m guardian, 0.25 mm and 0.25 µm film) with the column temperature ranging from 60 to 325 °C. Hydrogen was used as sample carrier gas. Data acquisition was performed using Agilent OpenLab CDS–Build 2.205.0.1344 (Agilent, Santa Clara, CA, USA.
Statistical analysis
Normality of data was determined by Shapiro-Wilk test. Statistical comparisons were performed by two-way ANOVA, followed by the Dunnett’s multiple comparisons test for all flow cytometry analyses. APE results were compared to control results, while APE + H2O2 results were compared to H2O2 data. Concerning the number of replicates, both the process and the analytical examinations were performed in duplicate. All statistical calculations were performed with the GraphPad Prism version 8.0 for Windows (GraphPad Software, San Diego, CA, USA). In all cases, p values lower than 0.05 were considered significant.
Results
Antioxidant extract restores yeast growth in the presence of hydrogen peroxide
The supplementation of the culture medium with antioxidant compounds is a strategy to overcome oxidative stress during fermentation (Burphan et al. 2018; Mendes et al. 2015; Qiu et al. 2019). The capacity of an antioxidant peptide-rich extract produced from Amyris Inc., Emeryville, USA, spent yeast (Oliveira et al. 2022b) to reduce oxidative stress in S. cerevisiae during 1 and 5 h of H2O2 exposure was studied. Hydrogen peroxide, H2O2 (2 and 5 mM), was used as stress inducer to increase ROS generation. AA was used as a positive control since it is an antioxidant known to reduce oxidative stress in yeast (Eigenfeld et al. 2021; Takagi 2021). Yeast growth was evaluated in drops spotted in agar plates after 1 and 5 h of incubation at 30 °C in liquid medium containing 2 or 5 mM H2O2 in the presence of 0.5 and 1 mM AA, and 0.5 g/L and 0.7 g/L APE (Fig. 2).
Yeast growth in the presence and absence of ascorbic acid (AA) or antioxidant peptide extract (APE) under hydrogen peroxide (H2O2) stress in agar plates, after 4 days of incubation at 30 °C. Cells were previously exposed to 2 mM H2O2 plus 0.5 mM AA, 1 mM AA, 0.5 g/L APE, and 0.7 g/L APE during 1 and 5 h (a and b, respectively). Cells were previously exposed to 5 mM H2O2 plus 0.5 mM AA, 1 mM AA, 0.5 g/L APE, and 0.7 g/L APE during 1 and 5 h (c and d). Three tenfold serial dilutions were done from an initial wOD = 1, and 4 µl of each dilution were spotted onto agar plates (wOD = 1, 10−1, 10−2, and 10.−3)
Yeast growth was improved by the addition of 0.5 and 1 mM AA, as well by 0.5 and 0.7 g/L APE after 1 and 5 h of exposure to 2 mM H2O2 (Fig. 2a and b, respectively). On the other hand, after 1 and 5 h of exposure to 5 mM H2O2, APE (0.5 and 0.7 g/L) led to a slight improvement in yeast growth (Fig. 2c and d). According to drops spotted in agar plates, under stress conditions, 0.5 and 0.7 g/L APE enhanced yeast growth, possibly by aiding cells to overcome oxidative stress through ROS reduction during incubation. To confirm this hypothesis, intracellular ROS and membrane integrity under H2O2 stress in the presence and absence of APE were analyzed.
Antioxidant compounds protect membrane integrity and decrease the level of intracellular ROS
Membrane integrity and ROS levels were measured by flow cytometry, after 1 and 5 h of H2O2 exposure in the different conditions tested (Fig. 3).
Membrane integrity and ROS production in yeast under hydrogen peroxide (H2O2) stress in presence of acetic acid (AA) or antioxidant peptide extract (APE). Membrane integrity was evaluated after 1 and 5 h of incubation with 2 mM H2O2 and 1 h with 5 mM H2O2 (a–c, respectively). Total ROS was also measured in fluorescence units relative to control (RFU) (d–f). The data represents the mean of two measurements for each condition, and error bars indicate standard deviations from the mean. Statistically significant differences were analyzed in all points using two-way ANOVA, followed by Dunnett’s multiple comparisons test (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05 vs H2O2)
Under H2O2 stress, membrane integrity was similar in the presence of antioxidant compounds, except in the condition 1 mM AA plus 2 mM H2O2, in which a significant high membrane integrity was observed after 5 h of exposure (Fig. 3a–c). On the other hand, under 2 mM H2O2 stress, supplementation of culture medium with APE and AA significantly improved yeast tolerance to oxidative stress (Fig. 3d and e). The addition of 0.5 g/L and 0.7 g/L of APE significantly decreased ROS levels by about 1.3 and 1.4-folds (17.08 RFU to 12.94 RFU and 11.97 RFU) after 1 h of incubation and 2.65 and 2.87-folds (14.62 RFU to 5.52 RFU and 5.1 RFU) after 5 h of exposure to H2O2, respectively. Thus, the higher reductions in ROS levels were promoted by 0.7 g/L APE. Moreover, 0.5 mM and 1 mM AA reduced ROS accumulation up to 1.68-fold (from 17.08 to 10.17 RFU) and 3.96-fold (from 17.08 to 4.31 RFU), respectively, after 1 h of 2 mM H2O2 exposure. After 5 h of incubation, in the presence of 2 mM H2O2, AA at 1 mM reduced ROS production from 14.62 to 6.24 RFU (2.0 times) and from 17.08 to 4.31 RFU (i.e., 4.92 times). Under 5 mM H2O2, ROS generation was high (117.62 RFU) and only 1 mM AA was capable to reduce ROS levels, to 87.16 RFU (Fig. 3f). Thus, in the presence of 2 mM H2O2, APE addition resulted in a significant reduction of ROS generated, which can be explained by its reported antioxidant properties (Oliveira et al. 2022b).
Impact of APE in small-scale fermentation
To study the effect of APE in small scale fermentation, shake-flask assays were conducted with 0.7 g/L APE as potential antioxidant and 2 mM H2O2 as stress inducer. Therefore, shake-flask fermentations were run using four different mediums, standard medium (control), medium with 0.7 g/L APE, medium with 2 mM H2O2, and medium with 0.7 g/L APE plus 2 mM H2O2. Results on yeast growth, farnesene concentration, membrane integrity, and intracellular ROS obtained during shake-flask fermentations are presented in Figs. 4 and 5.
Growth curve (washed optical density at 600 nm — wOD600) and sucrose (g/L), round dot dash, during 47 h of shake-flask fermentation in the absence (a) and presence (c) of stress factor (hydrogen peroxide — H2O2) and antioxidant peptide extract (APE). Farnesene (%) (b and d). Farnesene results of Fig. 4b and d were both normalized for the control result of Fig. 4b at 30.5 h, to demonstrate the effect of oxidative stress on farnesene production relatively to standard condition used in farnesene fermentations. Furthermore, Fig. 4b represents the effect of addition of APE without stress and Fig. 4d the effect of addition of APE in the presence of hydrogen peroxide. The data represent the mean of two independent replicates for each condition (control, 2 mM H2O2, 0.7 g/L APE, and 0.7 g/L APE + 2 mM H2O2), and error bars indicate standard deviations from mean
ROS production in yeast during shake-flasks fermentations under hydrogen peroxide (H2O2) stress with and without antioxidant peptide extract (APE) addition. a and b Total ROS were presented in relative fold units (RFU) variation during shake-flask fermentation in the absence or presence of 2 mM of H2O2 and 0.7 g/L APE. The data represents the mean of two independent replicates for each condition, and error bars indicate standard deviations from the mean. Statistically significant differences at each time point were determined using two-way ANOVA, followed by Dunnett’s multiple comparisons test (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05)
In flasks without stress, similar variation on wOD, farnesene concentration, and sucrose consumption were observed between control and APE conditions (Fig. 4a and b). However, in flasks with stress, addition of 0.7 g/L APE led to a significant improvement on yeast growth and farnesene production (Fig. 4c and d). Cell density during exponential phase in shake-flasks with 0.7 g/L of APE plus 2 mM H2O2 was almost double than in flasks with only 2 mM H2O2 (Fig. 4c). Sucrose consumption followed the same tendency, being higher in flasks with APE. In addition, farnesene concentration before sugar depletion, at 30.5 h, was 2.8-fold higher in shake-flasks with 0.7 g/L APE and 2 mM H2O2 than in shake-flasks with only 2 mM H2O2 (79.4% vs 28.5% of the maximum value in control condition) (Fig. 4d).
Membrane integrity was significantly improved by APE addition in the presence and absence of stress (Table 1). This improvement in shake-flaks under stress was higher at 5 and 23 h. Regarding oxidative stress, the reduction in ROS levels observed in the condition 0.7 g/L APE, in comparison to control, was not statistically significant (Fig. 5a). However, in the presence of 2 mM H2O2 (Fig. 5b), ROS levels were 1.2- to 3-fold significantly lower in shake-flask with 0.7 g/L APE addition than in shake-flask without antioxidants addition, during exponential phase of fermentation, respectively. The elevated standard deviation in the control at 23 h can be due to small variations in media volume or aeration between shake-flasks replicates (Fig. 5). In sum, the supplementation of S. cerevisiae fermentation with 0.7 g/L APE could improve yeast tolerance to oxidative stress and promote yeast growth, under demanding stress environment.
APE protects yeast from stress in bioreactor fermentation
Intracellular ROS generation in yeast can change with several conditions, namely, cellular aging, oxygen transfer rate, and ethanol concentration. Thus, in order to confirm if APE protective effect to yeast stress extends to larger fermentation scales, batch fermentations on 2.7 L bioreactors were performed.
As in shake-flask experiments, in batch bioreactor fermentations, the addition of 0.7 g/L APE into the culture medium alleviated the negative effect of the stress inducer H2O2. Relevant positive effects were observed on yeast growth, sugar consumption, and farnesene concentration (Figs. 6 and 7).
Growth curve (washed optical density at 600 nm — wOD600) and sucrose (g/L), over fermentation time for the conditions: control (no extract, no hydrogen peroxide (H2O2) added), antioxidant peptide extract (APE) at 0.7 g/L, 2 mM H2O2, and 2 mM H2O2 plus APE at 0.7 g/L (a and c). Farnesene concentration (%) relative to control at 34 h of fermentation for the conditions: control (no extract, no H2O2 added), APE extract at 0.7 g/L, 2 mM H2O2, and 2 mM H2O2 plus APE at 0.7 g/L (b and d). The data represents the mean of two measurements for each condition, and error bars indicate standard deviations from the mean
Membrane integrity and total ROS production in bioreactor fermentation under hydrogen peroxide (H2O2) stress and antioxidant peptide extract (APE) presence and absence. Membrane integrity in each condition tested (a and b). Variations on total ROS in conditions without stress addition (control and 0.7 g/L APE) (c) and conditions with stress (2 mM H2O2 and 0.7 g/L APE plus 2 mM H2O2) (d) over fermentation time. RFU represents the relative fold units determined in comparation with the cells analyzed in flow cytometer without the dihydrorhodamine 123 probe. The data represents the mean of two measurements for each condition, and error bars indicate standard deviations from the mean. Statistically significant differences at each time point were determined using two-way ANOVA, followed by Dunnett’s multiple comparisons test (****p < 0.0001; ***p < 0.001; **p < 0.01; *p < 0.05)
Bioreactors without stress factor (control vs 0.7 g/L APE) showed similar growth, sucrose consumption, and farnesene production during the entire fermentation (Fig. 6a and b). Nevertheless, in bioreactors under 2 mM H2O2, during the exponential phase (6 to 34 h), cell density was up to 1.5-fold higher in the presence of 0.7 g/L APE than in the absence of APE and sucrose consumption was faster (Fig. 6c). At 34 h of fermentation, bioreactor with 0.7 g/L APE plus 2 mM H2O2 achieved wOD600 of 36.75, consumed 52.81 g/L of sucrose, and had farnesene concentration of 70% of that in control condition (i.e., no extract, no stress induced), while bioreactor with 2 mM H2O2 only reached a wOD600 of 25.25, consumed 43.22 g/L of sucrose, and achieved farnesene concentration of 45% (Fig. 6c and d). Thus, the APE supplement led to an improvement of product formation of up to 1.6-fold when cells were subjected to stress. These observations can be attributed to a reduction in oxidative stress promoted by the APE, since the ROS were kept at lower levels when this natural antioxidant was present (Fig. 7).
In bioreactors without H2O2, APE addition only improved membrane integrity at 8 h of fermentation (Fig. 7a). However, in bioreactors under stress, membrane integrity was significantly increased by APE during exponential phase of fermentation (Fig. 7b). The range in membrane integrity in H2O2 condition was 96–89%, whereas in H2O2 plus APE condition that range was 96–94%. At 22 h of fermentation, membrane integrity was 5.5% higher in APE plus H2O2 compared to H2O2 condition.
In absence of stress, ROS levels were similar during most of the fermentation time, with no significant differences obtained, except at 22 h of fermentation where APE lead to a significant decrease in ROS levels (Fig. 7c). Although under stress condition a reduction in ROS levels between 1.4 and 1.7-fold was seen in most of the time points analyzed (Fig. 7d), this effect was only statistically significant at 8 and 30 h of fermentation. The higher difference in ROS values was at 8 h of fermentation from 9.27 RFUs, in 2 mM H2O2 bioreactor, to 6.40 RFUs in bioreactor with 2 mM H2O2 and 0.7 g/L APE.
According to the results, APE per se did not interfere with the fermentation, since when it was added to the culture medium, the obtained overall fermentation profile was similar to that on standard fermentation condition (i.e., control) (Figs. 6 and 7). Specifically, the APE, at the concentration of 0.7 g/L, did not promote significant differences on yeast growth, sugar consumption, and farnesene concentration over fermentation time in bioreactor batch fermentation. This confirms that the positive effect of APE seen under induced oxidative stress is based on its antioxidant properties and not on an additive effect.
Discussion
The biotechnology industry is gaining increasing importance in the production of relevant molecules to fulfill the needs of nowadays society while presenting a sustainable alternative to traditional industry such as petrochemical (Chen et al. 2020; Nandy and Srivastava 2018; Coelho et al. 2019; Stalidzans and Dace 2021). Cutting edge fermentation technology has enable the creation of different solutions, ranging from bacteria to eukaryotes, able to produce molecules of high value for different applications such as pharmaceutics, cosmetics, food and beverages, chemicals, plastics, and fuels (biofuels) (Chen et al. 2020; Mendes et al. 2015; Nandy and Srivastava 2018; Qiu et al. 2019). Given the importance of this technology for the transition to a sustainable and carbon-emission free paradigm, it is crucial to continue its development, optimizing processes and reducing production costs (Qiu et al. 2019; Stalidzans and Dace 2021; Takagi 2021; Walker and Basso 2020). On the other hand, this kind of industry also produces waste streams that should be taken into consideration and can be further valued and utilized in a circular economy approach (Faustino et al. 2021; Oliveira et al. 2022a,c; Pinto et al. 2015; San Martin et al. 2021).
The aim of this work was to study the impact of an antioxidant peptide extract (APE) isolated from industrial spent yeast to protect a farnesene producer yeast against stress in fermentation. Industrial fermentations are very demanding environments as yeasts are exposed to several stress factors which can be of physical, chemical, or biological origin (Deparis et al. 2017; Saini et al. 2018; Takagi 2021; Walker and Basso 2020). These factors have a negative impact in fermentation productivity yields; thus, it is of extreme importance to find solutions to overcome issues related to yeast stress. The APE used in this work was characterized elsewhere (Oliveira et al. 2022b), and given its origin and antioxidant properties, we wonder if it could protect yeast against an external stress, particularly, oxidative stress. The generation of ROS is a natural result of cellular aging, thus leading to cell senescence phenotype which hinder productivity (Eigenfeld et al. 2021).
To study this effect, yeast cells were exposed to different concentrations of hydrogen peroxide (H2O2 at 2 mM or 5 mM), commonly used as oxidative stress inducer, at different exposure periods (1 h or 5 h), in the presence or absence of APE. As positive control AA was used since it is commonly accepted as a potent antioxidant compound (Eigenfeld et al. 2021). Our results showed that APE was able to promote cell growth in the presence of the stress agent in a similar manner to AA (Fig. 2). This effect was more evident when using 2 mM of H2O2 for both 1 h and 5 h. In these conditions, membrane integrity was kept at acceptable values (> 90%) assuring that cells were still viable (Fig. 3 a and b). The use of 5 mM H2O2 for 5 h drastically disrupted the yeast growth by decreasing membrane integrity to very low levels (60%, data not shown) which unable the observation of the effects of both APE and AA. The protection against the stress agent by the capacity of APE to decrease the generation of intracellular ROS triggered by the presence of H2O2 was further evaluated. Following the same tendency of the positive control, APE lost its protective effect when cells were incubated with 5 mM H2O2. Previous studies also reported that sub-lethal H2O2 concentrations on yeast cells are lower than 0.4 mM, in which cells can adapt and tolerate, while concentrations higher than 1–2 mM can be lethal (Poljak et al. 2003). In a study with a 5-mM H2O2 exposure, yeast cell growth was drastically inhibited, and cell density decreased, as consequence of programmed cell death (apoptosis) or irreversible cell injury (necrosis) induced by H2O2 (Khan et al. 2005; Pedreño et al. 2002). Furthermore, upon exposure to 5 mM H2O2, the accumulation of protein carbonyls, indicators of oxidative proteins, increased (Khan et al. 2005). The results herein presented are in accordance with literature, which show that 5 mM H2O2 concentration (especially, for 5 h exposure) is strongly fatal for the yeast cells studied. Nevertheless, APE proved to decrease ROS production even at 5 h of exposure with 2 mM H2O2 indicating its potent antioxidant activity (Fig. 3e).
Afterwards, the potential antioxidant effect of APE on the fermentation performance of farnesene yeast producer in the presence of the oxidative stress factor was evaluated in small scale (shake-flasks) and bioreactor fermentations. In shake-flasks, the addition of 0.7 g/L APE to the culture medium in the presence of the stress factor improved yeast growth and farnesene production (Fig. 4 c and d). Membrane integrity was also improved by APE addition, especially at 5 and 23 h of fermentation (Table 1). Regarding ROS levels, APE promoted a decrease ranging from 1.2- up to threefold, until sugar depletion (Fig. 5b). The observed effects of APE under stress condition can be attributed to its antioxidant activity, as in other studies peptide extracts have shown to influence repair pathways against oxidative stress caused by H2O2 or UV radiation (Butylina et al. 2007; Mirzaei et al. 2019). Furthermore, the peptide extract used in the present study led to only 0.05 g/L of extra sugars and trace amounts of minerals in the culture medium, which was not considered significant for yeast growth and product titer. Of note, when APE was added to the normal condition, no relevant effects on yeast growth and farnesene production were observed. Moreover, total ROS levels in control condition were not significantly different from that observed in APE condition (Fig. 4a).
In bioreactor fermentation, the presence of APE in the medium acted similarly as in small-scale fermentations by presenting positive effects on yeast growth and sugar consumption while decreasing the levels of ROS generated by the presence of H2O2 (Figs. 6 and 7). In the presence of the stress factor, it was also observed that APE restored membrane integrity (Fig. 7b). Importantly, farnesene production was improved when medium was supplemented with APE in the presence of H2O2 (Fig. 6d). During large-scale (bioreactor) fermentation, oxidative stress has been generally associated with high levels of oxygen transfer rate (by means of high rates of aeration and agitation speeds) resulting in the production of ROS inside the cells (Siddiquee et al. 2019). By introducing 2 mM H2O2 into culture medium, this stress condition was elevated, as corroborated by ROS results in control condition of bioreactor batch experiment, where higher ROS values were reached than in shake-flasks (especially at 8, 22, and 30 h). Although ROS generation was superior in bioreactor experiment, 1.6-fold increment in farnesene production was observed in culture subjected to stress with APE supplement.
Overcoming oxidative stress is important not only to prevent cell aging but also to respond to other stress factors. Burphan and colleagues (2018) have demonstrated the importance of Cu/Zn-superoxide dismutase (SOD1) for yeast tolerance against different stresses occurring during industrial fermentation. The generation of H2O2 is a response to the oxidative stress caused by endogenous sources such as normal metabolism (Ceriello et al. 2016) but also of exogenous origins such high extracellular sugar concentration (Semchyshyn et al. 2011) or heat stress (Davidson and Schiestl 2001). The extraction and use of antioxidant peptides from different origins for evaluation of yeast protection against oxidative stress have been evaluated (San Martin et al. 2021; Coelho et al. 2019). For example, four of the eight antioxidant peptide fractions of chia (0.5 g/L) tested by Coelho et al. (2019) promoted an increase of yeast survival rate (16–27%) in the presence 0.75 mM H2O2. However, to best of our knowledge, this is the first report of the use of antioxidant peptides of yeast origin to improve fermentation and yeast performance with industrial relevance. The APE used in this work has an antioxidant potential around 500 µmol 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) /mg protein with 2,2′-azinobis(3-ethyl-benzothiazoline-6-sulphonate)-radical (ABTS+) scavenging assay but the mechanism of oxidative protection might involve other mechanisms than just ROS direct scavenging, such as inhibition of lipid oxidation (Oliveira et al. 2022b). Xu and colleagues have shown that of the 20 essential amino acids, 7 have antioxidant activity namely tryptophan, methionine, histidine, lysine, cysteine, arginine, and tyrosine (Xu et al. 2017). The APE used has 5 of these 7 amino acids comprising approximately 15% of the total protein content (Oliveira et al. 2022b). Furthermore, it is well known that arginine, a nutritionally important amino acid, is involved in stress resistance in various organisms (Ji et al. 2020; Nishimura et al. 2010). Thus, it might be hypothesized that the APE is not only scavenging ROS by direct action but also may stimulate other yeast stress defense mechanisms.
Overall, the results herein presented demonstrate that the supplementation of farnesene fermentation process with peptides recovered from industrial spent yeast is a promising strategy to reduce ROS generation, and thus, improve yeast tolerance to oxidative stress while increasing biomolecule productivity. Since APE was obtained from Amyris Inc., Emeryville, CA, USA, fermentation waste, its application to reduce yeast oxidative stress in industrial fermentation may allow to increase process profitability, while promoting both, the valorization of spent yeast waste stream, and the improvement of yeast performance and productivity. Also important, from a circular economy perspective, the valorization of waste streams is also a valuable contribution towards sustainable and eco-friendly solutions for the biotechnology industry. To the best knowledge of the authors, this is the first time that this type of peptide extract is used to overcome oxidative stress in farnesene fermentation.
Data availability
Not applicable.
References
Auesukaree C (2017) Molecular mechanisms of the yeast adaptive response and tolerance to stresses encountered during ethanol fermentation. J Biosci Bioeng 124(2):133–142. https://doi.org/10.1016/j.jbiosc.2017.03.009
Benjamin KR, Silva IR, Cherubim JP, McPhee D, Paddon CJ (2016) Developing commercial production of semi-synthetic artemisinin, and of β-farnesene, an isoprenoid produced by fermentation of brazilian sugar. J Braz Chem Soc Society 27(8):1339–1345
Burphan T, Tatip S, Limcharoensuk T, Kangboonruang K, Boonchird C, Auesukaree C (2018) Enhancement of ethanol production in very high gravity fermentation by reducing fermentation-induced oxidative stress in Saccharomyces cerevisiae. Sci Rep 8(1):13069. https://doi.org/10.1038/s41598-018-31558-4
Butylina S, Shataeva LK, Nyström M (2007) Separation of nucleoprotein complexes with antioxidant activity from yeast Saccharomyces cerevisiae. Sep Purif Technol 53:64–70
Carsanba E, Pintado M, Oliveira C (2021) Fermentation strategies for production of pharmaceutical terpenoids in engineered yeast. Pharmaceuticals 14(4):295
Carvalho LC, Oliveira ALS, Carsanba E, Pintado M, Oliveira C (2022) Phenolic compounds modulation in β-farnesene fed-batch fermentation using sugarcane syrup as feedstock. Ind Crops Prod 188:115721. https://doi.org/10.1016/j.indcrop.2022.115721
Ceriello A, Testa R, Genovese S (2016) Clinical implications of oxidative stress and potential role of natural antioxidants in diabetic vascular complications. Nutr Metab Cardiovasc Dis 26(4):285–292. https://doi.org/10.1016/j.numecd.2016.01.006
Chen R, Yang S, Zhang L, Zhou YJ (2020) Advanced strategies for production of natural products in yeast. iScience 23(3):100879. https://doi.org/10.1016/j.isci.2020.100879
Coelho M, de Araujo Aquino S, Machado Latorres J, de las Mercedes Salas-Mellado M (2019) In vitro and in vivo antioxidant capacity of chia protein hydrolysates and peptides. Food Hydrocoll 91:19–25. https://doi.org/10.1016/j.foodhyd.2019.01.018
Davidson JF, Schiestl RH (2001) Mitochondrial respiratory electron carriers are involved in oxidative stress during heat stress in Saccharomyces cerevisiae. Mol Cell Biol 21(24):8483–8489. https://doi.org/10.1128/mcb.21.24.8483-8489.2001
Deparis Q, Claes A, Foulquié-Moreno MR, Thevelein JM (2017) Engineering tolerance to industrially relevant stress factors in yeast cell factories. FEMS Yeast Res 17(4):fox036. https://doi.org/10.1093/femsyr/fox036
Eigenfeld M, Kerpes R, Becker T (2021) Understanding the impact of industrial stress conditions on replicative aging in Saccharomyces cerevisiae. Front Fungal Biol 2:665490. https://doi.org/10.3389/ffunb.2021.665490
Faustino M, Durão J, Pereira CF, Pintado ME, Carvalho AP (2021) Mannans and mannan oligosaccharides (MOS) from Saccharomyces cerevisiae — a sustainable source of functional ingredients. Carbohydr Polym 272:118467. https://doi.org/10.1016/j.carbpol.2021.118467
Ji H, Xu K, Dong X, Sun D, Peng R, Lin S, Zhang K, Jin L (2020) Transcriptional profiling reveals molecular basis and the role of arginine in response to low-pH stress in Pichia kudriavzevii. J Biosci Bioeng 130(6):588–595. https://doi.org/10.1016/j.jbiosc.2020.07.014
Khan MA, Chock PB, Stadtman ER (2005) Knockout of caspase-like gene, YCA1, abrogates apoptosis and elevates oxidized proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 102(48):17326–17331. https://doi.org/10.1073/pnas.0508120102
Li K, Zhang J-W, Liu C-G, AamerMehmood M, Bai F-W (2020) Elucidating the molecular mechanism of TEMPOL-mediated improvement on tolerance under oxidative stress in Saccharomyces cerevisiae. Chem Eng Sci 211:115306. https://doi.org/10.1016/j.ces.2019.115306
Meadows AL, Hawkins KM, Tsegaye Y, Antipov E, Kim Y, Raetz L, Dahl RH, Tai A, Mahatdejkul-Meadows T, Xu L, Zhao L, Dasika MS, Murarka A, Lenihan J, Eng D, Leng JS, Liu C-L, Wenger JW, Jiang H, Chao L, Westfall P, Lai J, Ganesan S, Jackson P, Mans R, Platt D, Reeves CD, Saija PR, Wichmann G, Holmes VF, Benjamin K, Hill PW, Gardner TS, Tsong AE (2016) Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 537(7622):694–697. https://doi.org/10.1038/nature19769
Mendes V, Vilaça R, de Freitas V, Ferreira PM, Mateus N, Costa V (2015) Effect of myricetin, pyrogallol, and phloroglucinol on yeast resistance to oxidative stress. Oxid Med Cell Longev 2015:782504. https://doi.org/10.1155/2015/782504
Mirzaei M, Mirdamadi S, Safavi M (2019) Antioxidant activity and protective effects of Saccharomyces cerevisiae peptide fractions against H2O2-induced oxidative stress in Caco-2 cells. J Food Meas Charact 13:2654–2662
Nandy SK, Srivastava RK (2018) A review on sustainable yeast biotechnological processes and applications. Microbiol Res 207:83–90. https://doi.org/10.1016/j.micres.2017.11.013
Nishimura A, Kotani T, Sasano Y, Takagi H (2010) An antioxidative mechanism mediated by the yeast N-acetyltransferase Mpr1: oxidative stress-induced arginine synthesis and its physiological role. FEMS Yeast Res 10(6):687–698. https://doi.org/10.1111/j.1567-1364.2010.00650.x
Oliveira AS, Ferreira C, Pereira JO, Pintado ME, Carvalho AP (2022) Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: an overview. Int J Biol Macromol 208:1116–1126. https://doi.org/10.1016/j.ijbiomac.2022.03.094
Oliveira CM, Horta B, Leal T, Pintado M, Oliveira CSS (2022) Valorization of spent sugarcane fermentation broth as a source of phenolic compounds. Processes 10(7):1339
Oliveira AS, Pereira JO, Ferreira C, Faustino M, Durão J, Pintado ME, Carvalho AP (2022b) Peptide-rich extracts from spent yeast waste streams as a source of bioactive compounds for the nutraceutical market. Innov Food Sci Emerg Technol 81:103148.https://doi.org/10.1016/j.ifset.2022.103148
Pedreño Y, Gimeno-Alcañiz JV, Matallana E, Argüelles JC (2002) Response to oxidative stress caused by H2O2 in Saccharomyces cerevisiae mutants deficient in trehalase genes. Arch Microbiol 177(6):494–499. https://doi.org/10.1007/s00203-002-0418-2
Pinto M, Coelho E, Nunes A, Brandão T, Coimbra MA (2015) Valuation of brewers spent yeast polysaccharides: a structural characterization approach. Carbohydr Polym 116:215–222. https://doi.org/10.1016/j.carbpol.2014.03.010
Poljak A, Dawes IW, Ingelse BA, Duncan MW, Smythe GA, Grant CM (2003) Oxidative damage to proteins in yeast cells exposed to adaptive levels of H2O2. Redox Rep 8(6):371–377. https://doi.org/10.1179/135100003225003401
Qiu X, Zhang J, Zhou J, Fang Z, Zhu Z, Li J, Du G (2019) Stress tolerance phenotype of industrial yeast: industrial cases, cellular changes, and improvement strategies. Appl Microbiol Biotechnol 103(16):6449–6462. https://doi.org/10.1007/s00253-019-09993-8
Saini P, Beniwal A, Kokkiligadda A, Vij S (2018) Response and tolerance of yeast to changing environmental stress during ethanol fermentation. Process Biochem 72:1–12. https://doi.org/10.1016/j.procbio.2018.07.001
San Martin D, Ibarruri J, Iñarra B, Luengo N, Ferrer J, Alvarez-Ossorio C, Bald C, Gutierrez M, Zufía J (2021) Valorisation of brewer’s spent yeasts’ hydrolysates as high-value bioactive molecules. Sustainability 13(12):6520
Semchyshyn HM, Lozinska LM, Miedzobrodzki J, Lushchak VI (2011) Fructose and glucose differentially affect aging and carbonyl/oxidative stress parameters in Saccharomyces cerevisiae cells. Carbohydr Res 346(7):933–938. https://doi.org/10.1016/j.carres.2011.03.005
Siddiquee K, Zhao C, Stemler MA, Zeck B, Fishpaugh JR, Allen SP (2019) Cell-culture growth conditions resulting in the oxidation of a recombinant antigen-binding fragment. Bioresour Bioprocess 6(1):34. https://doi.org/10.1186/s40643-019-0270-8
Stalidzans E, Dace E (2021) Sustainable metabolic engineering for sustainability optimisation of industrial biotechnology. Comput Struct Biotechnol J 19:4770–4776. https://doi.org/10.1016/j.csbj.2021.08.034
Takagi H (2021) Molecular mechanisms and highly functional development for stress tolerance of the yeast Saccharomyces cerevisiae. Biosci Biotechnol Biochem 85(5):1017–1037. https://doi.org/10.1093/bbb/zbab022
van Hoek P, de Hulster E, van Dijken JP, Pronk JT (2000) Fermentative capacity in high-cell-density fed-batch cultures of baker’s yeast. Biotechnol Bioeng 68(5):517–523. https://doi.org/10.1002/(SICI)1097-0290(20000605)68:5%3c517::AID-BIT5%3e3.0.CO;2-O
Walker GM, Basso TO (2020) Mitigating stress in industrial yeasts. Fungal Biol 124(5):387–397. https://doi.org/10.1016/j.funbio.2019.10.010
Xu N, Chen G, Liu H (2017) Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 22(12):2066. https://doi.org/10.3390/molecules22122066
Funding
Open access funding provided by FCT|FCCN (b-on). This work was funded by the Amyris Bio Products Portugal Unipessoal Lda and Escola Superior de Biotecnologia–Universidade Católica Portuguesa through Alchemy project — Capturing high value from industrial fermentation bio products (POCI-01–0247-FEDER-027578).
Author information
Authors and Affiliations
Contributions
Conceptualization: C. O., E. C., J. A.-S.; funding acquisition: M. P.; investigation: A. L., A. S. O., C. F., J. O. P.; supervision: C. O., E. C., J. A.-S., A. P. C.; writing—original draft: A. L., J. A.-S.; writing—review and editing: A. L., C. O., E. C., J. A.-S., A. P. C., A. S. O., C. F., J. O. P. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
A US patent with provisional application number 63/429,893 protecting the use of APE in yeast fermentation disclosed in this manuscript has been filed by the following authors: A. Lopes, E. Carsanba, A.S. Oliveira, A.P. Carvalho, and C. Oliveira. The authors J. Azevedo-Silva, M. Pintado, C. Ferreira and J. Odila Pereira have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lopes, A., Azevedo-Silva, J., Carsanba, E. et al. Peptide extract from spent yeast improves resistance of Saccharomyces cerevisiae to oxidative stress. Appl Microbiol Biotechnol 107, 3405–3417 (2023). https://doi.org/10.1007/s00253-023-12514-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-023-12514-3