Abstract
Japanese encephalitis virus (JEV) is one of the leading causes of epidemic encephalitis in South Asian countries. Due to the short-term viremia, detecting IgM antibodies by ELISA is treated as the front-line diagnostic assay. Co-circulation and multiple exposures to antigenically cross-reactive flaviviruses in India pose a challenge in serodiagnosis. Replacing the whole virus antigen currently used in the JE IgM detection kits (ELISA) may improve the specificity and sensitivity of the existing JE MAC ELISA kits. For this purpose, we developed a stably transfected cell clone, BHK-IE6, which expresses a high amount of VLPs up to 37 µg/ml and is consistent in expression up to 40 passages. For the expression of VLPs in the secretory form, we cloned the JEV G-I prM-E coding gene along with the C-terminal signal sequence of capsid protein in the BHK-21 cells using the pcDNA3.1 + mammalian expression vector. The immune assays performed demonstrated its immune reactivity equivalent to the parental JEV strain. Simultaneously performed ELISAs using the whole virus antigen and newly developed antigen gave comparable results for JE positive and negative samples, which established the utility of developed JEV E-VLP as an antigen. Reduced cross-reactivity and increased specificity were observed when tested with dual positive sera for anti-JEV and DENV antibodies. These findings confirm the efficiency and reliability of newly developed recombinant E-VLP antigen expressed by the BHK-IE6 cell clone as an antigen in serodiagnostic assays. The implementation and progress in developing cross-reactivity-reduced antigens would improve serodiagnosis and disease burden estimates of flavivirus infection.
Key points
• pcDNA3.1/JE-Sig-prM-E plasmid transfected BHK-21 cells stably express VLPs.
• Sodium butyrate induction enhanced the extracellular expression of VLPs.
• Application of JEV-E VLPs increases the specificity of JE IgM ELISA.
Similar content being viewed by others
Introduction
Japanese encephalitis (JE) caused by the JE virus (JEV) represents the significant etiology of pediatric encephalitis and disability. An estimated three billion people from Asian and Western Pacific regions live in JE endemic areas (WHO, 2015). Despite the extensive JE vaccination efforts, JEV is responsible for an estimated 67,900 clinical cases, with an average of 13,600 to 20,400 deaths per year. JE clinical symptoms range from mild febrile illness to acute meningomyeloencephalitis leading to 20–30% fatalities, out of which 30–50% survivors suffer long-term neurologic or psychiatric sequelae (CDC, 2013). JEV is a member of the Flaviviridae family, transmitted by Culex mosquitoes in an enzootic transmission cycle involving birds, swine, and other non-avian vertebrate hosts. Based on genetic analysis of partial or full-length sequence, the globally isolated JEV strains are classified into five genotypes: GI to GV (Uchil and Satchidanandam 2001; Schuh et al. 2014). During the last 2 decades, a dramatic change in the JE epidemiology has been documented as JEV GI was gradually introduced in most Asian countries and dominated by displacing the earlier prevalent GIII strains (Sarkar et al.2012; Schuh et al. 2014).
Flavivirus envelope (E) glycoprotein poses different biological functions, including induction of the neutralizing antibodies, protective immunity, virulence, and cell tropism, making it a major target for antiviral immunity (Ali and Igarashi 1997). JEV RNA is rarely detectable in cerebrospinal fluid (CSF) or serum samples due to short-term viremia. Hence, JE diagnosis relies mainly on detecting IgM antibodies developed against the JEV in acute CSF or serum samples. Accordingly, the World Health Organization (WHO) anti-JEV IgM ELISA is a front-line method for JEV diagnosis (Martin et al. 2000; WHO, 2006). However, co-circulation of multiple antigenically cross-reactive flaviviruses in the region may result in a false diagnosis. Such a compromised outcome observed in endemic areas raises significant public health concerns for the intervention programs that need careful interpretation (Mansfield et al. 2017; Johnson et al. 2016). Thus, developing new or refinement of existing tools for robust, specific, and sensitive diagnostic during early infection is necessary to implement effective control measures.
JE MAC ELISA detects anti-JEV IgM antibodies early in the course of infection, but its specificity and sensitivity depend on the purity and type of antigen preparations used to capture the IgM antibodies in clinical samples (Cuzzubbo et al. 1999). The commercially available JEV IgM ELISA kits based on the whole virus show significant cross-reactivity in regions endemic to JE and dengue and have a risk of handling high titer virus (Ravi et al. 2006). The use of immunodominant envelope protein in place of complete virion as an antigen in the assay will help to reduce flavivirus cross-reactivity (Innis et al. 1989). In India, only a few JE IgM ELISA kits are available commercially, like the IgM ELISA kit manufactured by ICMR-National Institute of Virology (NIV), Pune, which employs cell culture grown inactivated JEV GIII strain as an antigen and a flavivirus-specific monoclonal antibody to detect the antigen-IgM antibody complex (Kedarnath et al. 1986; Gadkari and Shaikh, 1984). Field studies performed using various JE IgM kits concluded their limitations on specificity and the use of specimens that need improvement (Lewthwaite et al. 2010). This study explored the use of VLPs as an antigen generated by expressing the E protein-coding region of JEV GI strain (0,945,054) along with the precursor membrane protein (prM) and a signal sequence located at the C-terminus of the nucleocapsid coding region in the mammalian expression system. The signal sequence is essential for generation, self-assembly, antigenic integrity, and extracellular release of recombinant protein as virus-like particles (VLPs) mimicking the JEV structure (Taylor et al. 2016). We have observed minimized false positivity with samples from dengue cases. Furthermore, the newly developed IgM-ELISA assay was standardized using well-characterized reference panel sera from JE positive and negative cases and compared with the existing JE IgM ELISA kit.
Materials and methods
Cloning of JEV envelope glycoprotein
Viral RNA from JEV G I strain (0,945,054-Human)-infected culture supernatant was extracted, and RT-PCR amplified using forward primer JE-F390-KpnI and reverse primer JE-R2477-EcoRI, for cloning of the JEV envelope protein-coding region, along with C-terminal signal (Sig) peptide of nucleocapsid membrane protein-coding regions (genomic location 390 to 2477 nt; GenBank Accession # MT859415). The PCR-purified product was cloned in pcDNA3.1 + mammalian expression vector and transformed into Escherichia coli TOP 10 chemically competent cells as per standard protocol (Maniatis et al.1982). The recombinant plasmid carrying JEV insert was isolated from transformed bacteria and confirmed for the insert DNA by PCR amplification and sequencing with T7 forward and BGH reverses primers (Table 1).
Transient expression of recombinant E protein
Baby hamster kidney cells (BHK-21) maintained in Minimum Essential Medium (MEM, Sigma-Aldrich) supplemented with 10% fetal bovine serum (FBS: Gibco), penicillin (100 U/ml), and streptomycin (100 µg/ml) were transfected with Lipofectamine 3000 transfection kit (Thermo Fisher Scientific) following the manufacturer’s instructions. Briefly, the cells at 0.25 × 106/ml seeding density were transfected with 3 µg of recombinant plasmid using P3000 enhancer (6 µl) reagent and Lipofectamine 3000 (6 µl) and incubated at 37 °C with 5% CO2 for expression analysis up to 96 h post-transfection. After every 24 h of transfection, IFA and ELISA were performed to confirm the expression and secretion in cells and cell supernatant, respectively.
Establishment of cells stably expressing JEV E protein
The transfected cells were seeded to obtain single-cell cultures by limiting dilutions in a 96-well microplate. The growth media was supplemented with 1000 µg/ml of G418 (Sigma-Aldrich) to allow only positively transfected cells to grow. Wells with single-cell colonies were marked and propagated to larger culture flasks and maintained under G418. Clones with higher OD were labeled according to their location in 96-well plates and cryo-preserved. At the end of every fifth passage, the presence of JEV insert in cellular DNA was confirmed by PCR amplification and sequencing using the insert specific primers (Table.1).
Induction of recombinant protein expression
One of the stable clones (BHK-IE6) was induced with different concentrations (ranging from 0 to 36 mM/ml) of sodium butyrate (C4H7NaO2) (Sigma), and the level of recombinant protein expression was monitored. Briefly, a monolayer of BHK-IE6 clone grown in 24-well plate was induced with different concentrations of sodium butyrate, and levels of expression were verified by ELISA.
Immunofluorescence assay (IFA)
The immunofluorescence assay was carried out as described by Gangwar et al. (2011) to detect recombinant JEV E protein expression in transfected BHK-21 cells (Gangwar et al. 2011). Immune mice serum generated against the parental JEV strain was used as a primary source of antibodies.
Antigen capture ELISA
Antigen capture ELISA was performed to detect recombinant protein expression in the cell supernatant as previously described (Cecilia et al. 1988; Gangwar et al. 2011). Briefly, the supernatant of transfected cells was added to the wells precoated with mouse anti-JEV-specific monoclonal antibody (HS-3: An India strain JE-733913 was used for the MAbs development, and HS-3 is JE strain specific and specifically detects the E-II of the E glycoprotein). The JEV E antigen captured to the solid phase was detected with biotin-conjugated HS-3 antibodies and subsequently with avidin horseradish peroxidase using TMB as liquid substrate (Dako Corp Carpinteria, California). Clone supernatant with a signal-to-noise (S/N) ratio of more than 2 was considered positive (Gangwar et al. 2011).
SDS-PAGE and western blotting
The protein samples prepared from transfected cell supernatants were separated on 10% (SDS-PAGE) and analyzed by western blotting (Laemmli, 1970). Briefly, the SDS-PAGE separated proteins were transferred on the nitrocellulose membrane and, after blocking the membrane, were exposed to HS-3 antibodies (1:20 diluted in TBS). After extensive washing with 1XTBS-T(0.1%Tween-20), the blot was incubated with goat anti-mouse horseradish peroxidase (HRP) conjugate at a dilution of 1:3000 for 1 h at RT. Further, the blot was developed with DAB and hydrogen peroxide (30%H2O2) in TBS (Towbin et al. 1979).
Protein extracted from 100 µl of cell supernatant was harvested at different passage numbers of the transfected cell line (BHK-IE6), and an increasing concentration of BSA (1 to 5 µg) was separated on 10% SDS-PAGE. The CBB-stained SDS-PAGE was analyzed through the ImageJ software (https://imagej.en.lo4d.com/), and a standard graph of BSA concentration against the pixel density of the bands was plotted (Alonso Villela et al. 2020). The pixel density of E protein obtained from each band was plotted on the standard graph, and the amount of E protein per 100 µl of cell supernatant was estimated.
Purification and TEM analysis of VLPs
The clarified cell supernatant of the BHK-IE6 cells containing JE-VLPs was precipitated using 12% polyethylene glycol (PEG; molecular mass, approximately 6,000 Da) and 0.5 M NaCl. Briefly, the cell supernatant along with PEG and NaCl was incubated at 4 °C with constant stirring for 2 h and centrifuged at 14,000 rpm for 45 min to get the pellet. The pellet was resuspended in TNE buffer and filtered through 100 kDa MWCO filters (Merck) to remove any other cellular contaminants. Purified JE VLPs were examined under the transmission electron microscope (TEM) Tecnai 12 BioTwin™ (FEI Company, the Netherlands), and images were captured using a side-mounted 2 k × 2 k CCD camera (Mega view III, Olympus, Japan), as described by Gangodkar et al. (2010).
Characterization of the BHK-IE6 clone
The expression stability of the BHK-IE6 clone and the impact of multiple harvesting of expressed protein were evaluated. The cell supernatant at every 5th passage and soup harvested from a single monolayer at every 72 h multiple times (n = 4) was analyzed by antigen capture ELISA. The cells were cryopreserved using standard protocol for the long-term storage of the BHK-IE6 clone (Phelan and May 2016) and stored in liquid nitrogen.
Comparative IgM capture ELISA using JE-VLPs and commercial kit antigen
JE IgM capture ELISA was developed using the expressed E protein as an antigen to detect anti-JEV IgM antibodies in clinical specimens. Standard panel of human sera pre-tested to be JE IgM positive (n = 10), JE IgM indeterminate (n = 10), JE IgM negative (n = 10), dengue IgM positive (n = 10), dengue IgM negative (n = 10), and dual positive for IgM antibodies to JE and dengue were used as test samples to evaluate the specificity and utility of the newly developed VLP antigen-based assay. Briefly, IgM antibodies from patient sera were captured on anti-human IgM-coated wells, and the antigen–antibody complex was probed with biotin-labeled HS-3 monoclonal antibodies (MAb) followed by avidin-HRP and TMB as substrate. The reaction was terminated by adding 1 N H2SO4, and the optical density (OD) value was measured at 450 nm. JE MAC ELISA based on conventional inactivated antigen and JEV E antigen-based IgM ELISA were simultaneously performed to test the serum samples. Briefly the virus stock was inactivated by adding 0.5% glycine powder (Sigma-Aldrich) and 1:2000 final concentration of formalin (Sigma-Aldrich) and stirring the mixture at 4 °C for 48 h; residual formalin was removed by keeping the inactivated virus stock in fume hood for 1 h. One hundred microliters of JEV recombinant E protein (3 µg/well) was served as antigen. Virus stock of JE G-I strain 0,945,054 with 1 × 106 PFU/ml titer was served as positive control, and non-infected BHK-21 cells supernatant were served as negative antigen controls in the assay. JEV GI strain 0,945,054 used in this study was originally isolated from human serum sample of hospitalized case. As per the NIV Virus Registry, the sample ID 0,945,054 denotes the year 2009 followed by the human clinical specimen’s number collected in 2009. Further, the test results were interpreted according to the JE IgM ELISA kit instructions, and the results were compared with each other. As per the JE IgM EISA protocol, test sera giving OD of ≤ negative control by a factor of 3 were considered negative, while sera giving OD of ≥ negative control by a factor of 5 were considered positive. The newly developed test was considered valid only if the OD of the negative control is ≤ 0.8 and the OD of positive control was ≥ 5 times the OD of negative controls.
Results
Cloning of JEV envelope glycoprotein
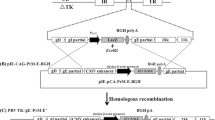
RT-PCR of JEV RNA genome with forward JE-F390-KpnI and reverse JE-R2477-EcoRI primer resulted in amplification of a 2121 bp DNA fragment ( Sig-prM-E) containing KpnI site upstream to the Kozak sequences and initiation codon at 5′ end and stop codon downstream to the EcoRI site at its 3′ end (Fig. 1). Ligation of the fragment and pcDNA3.1 + plasmid (5.4 bp) after sequential digestion with KpnI and EcoRI resulted in the construction of a recombinant pcDNA3.1/JE-Sig-prM-E (7506 bp) plasmid carrying insert DNA (Fig. 1). DNA sequencing of the insert, amplified from an isolated plasmid of transformed E. coli, confirmed directional, in-frame cloning of the JEV Sig-prM-E fragment in pcDNA 3.1.
Amplification of E gene of JEV. Lane 1: 2.1 kb PCR product as an insert. Lane 2: linearized vector—5.4 kb (pcDNA 3.1 + digested with KpnI and EcoRI restriction enzymes). Lane 3: linearized recombinant plasmid—7.5 kb containing vector + insert. Lane 4: 1 Kb + DNA extension ladder (Life Technologies, USA)
Stable expression of JEV envelope protein
As evident from IFA, the JEV E protein expression was observed in > 40% of the transfected BHK-21 cells by the end of 24 h and continued until 96 h of transfection (Fig. 2). By limiting dilution and propagation of transfected cells under G418 pressure, we achieved 39 cell clones expressing JEV E protein. However, we found a loss of IFA positivity in 19 clones at the six serial passages. Furthermore, IFA and antigen-capture ELISA confirmed the expression and extracellular secretion of JEV E protein by the clones obtained. One of the BHK-21 clones (BHK-IE6) was further expanded in a higher capacity culture flask under G418 selection pressure. Comparatively similar morphology was observed between BHK-21 and BHK-IE6 clones where no polykaryocytes formation was found (Fig. 3).
Immunofluorescence assay for detection of JEV E glycoprotein in transfected BHK- 21 cells and BHK-IE6 clone obtained through single cell dilution of transfected cells. a BHK-21 cells transfected with recombinant plasmid; b single cell clone BHK–IE6; c BHK-21 cells infected with JE 0,945,054; d mock-transfected BHK-21 cells. The polyclonal antibodies against the JEV GI were used as primary antibody while goat-anti-mouse antibodies conjugated with FITC were used as secondary antibodies. The images were taken using Floid™ cell imaging station (Invitrogen) at 20 × magnification with the scale bar = 100 µm
Induction of JEV E protein expression
As established by antigen-capture ELISA performed on cell-free supernatant, a gradual increase in JEV E protein expression by the stably transfected BHK-IE6 clone with increasing concentrations of sodium butyrate was observed, and maximum amount of expressed protein 37 µg/ml was observed at 72 h post-induction with 28 mM of sodium butyrate after addition at 24 h post-seeding. But a decline in E protein expression was documented at higher concentrations (32–36 mM) of sodium butyrate (Fig. 4). Western blot analysis further established the expression and reactivity of the 54 kDa JEV E protein (Fig. 5).
Enhanced expression of E protein by sodium butyrate induction (Multiple conc. of NaBu (0–36 mM) used for induction and culture supernatants harvested after 24, 48, and 72 h post-induction were analyzed for the E protein concentration. Maximum amount of protein was detected after 72 h of induction using 28 mM of sodium butyrate by antigen-capture ELISA to detect the amount of E protein expressed
Coomassie brilliant blue (CBB) stained SDS-PAGE and Western blot analysis of the VLPs expressed in stably transfected cell clone BHK-IE6. Lanes 1, 2, and 3: purified cell supernatant of stably transfected cell clone BHK-IE6. Lane 4: cell supernatant of mock transfected BHK-21 cells. Lane 5: standard protein molecular weight markers (a). The blot was probed with anti-JEV HS-3 monoclonal antibody. Lane 1, standard protein molecular weight markers; lane 2, JE virus infected cell soup; lane 3, VLPs expressing BHK-IE6 cell soup; lane 4, negative control BHK-21 cell soup (b)
Characterization of the BHK-IE6 clone
Expression levels of the BHK-IE6 clone was found to be stable up to 30 passages as the level of expression was unaltered. As described earlier, using the ImageJ software and the CBB-stained SDS-PAGE, we recorded increased JEV E protein expression from passage 1 to 10. At 5th and 10th passage level, 26 and 33 µg of E protein were estimated from 1 ml of cell supernatant. From passage level 10 to 25, equal amount of E protein expression was observed (33–34 µg/ml). However, E protein expression declined after 25 passages up to 10 µg/ml at the 40th passage (Fig. 6a). In a single passage of BHK-IE6, culture fluid harvested post-induction with sodium butyrate showed expression of 32–35 µg of JEV E protein in the 1st and 2nd harvests (72 and 144 h), which was slightly declined to 25 µg at 3rd harvest (216 h), while on 4th harvest (288 h), it reached to 12 µg (Fig. 6b). Even after 6 months of storage by cryopreservation and revival, the expression levels did not alter.
a Effect of long-term passage on production of JEV-VLP antigen by BHK-IE6 clone. IE6 cells were passaged every 3 days in a 75 cm2 flask, and the amount of antigen produced by IE6 cells from the different passages was analyzed using ELISA; b the culture supernatant of confluent BHK-IE6 cells of passage no. 39 was refreshed every 72 h, and the amount of JEV-VLP antigen in culture supernatants was determined by ELISA
Electron microscopic analysis of expressed JEV E protein
Transmission electron microscopy imaging of the negative-stained recombinant JEV E protein showed spherical morphologic features of flaviviruses (Fig. 7b), suggesting its appearance of virus-like particles. The variable size of VLPs ranged from 30 to 50 nm. The spherical structures taken up by the purified JEV E protein confirmed their assembly as VLP.
Comparative IgM capture ELISA using JE-VLPs and commercial kit antigen
The newly developed JE IgM ELISA based on expressed JEV E protein as an antigen was assessed for specificity using a panel of pre-characterized human sera. The OD values obtained for the JE positive and negative sera by the newly developed JEV E antigen-based IgM ELISA were equivalent to the conventional JE IgM ELISA (Supplementary Table.1). The comparative analysis of OD values obtained from newly developed ELISA and conventional ELISA for each sample showed the correlation coefficient of 0.997 (Fig. 8), confirming the utility of newly developed JEV E protein-based antigen in place of conventional whole virus antigen. Out of the 10 indeterminate samples, all yielded to be negative except 1 sample. (Table S1). Out of the 10 dual positive samples selected, only 2 sera were found to be positive (Table S2). The standard panel of dengue IgM positive and negative human sera were tested negative (Table S3). The comparative analysis between VLP antigen and conventional inactivated antigen was done in triplicates using a standard human sera panel to validate the new assay developed.
Discussion
JE is one of the important public health issues endemic to most parts of India except the arid and highland regions. Virus isolation or genome detection of viral RNA in clinical samples is treated as the “gold standard” of diagnosis, but short-term viremia and low titer of the virus during infection limit their applications. Hence, detection of IgM antibodies by MAC-ELISA in clinical specimens is widely recommended by WHO as the front-line screening assay for JE diagnosis (WHO, 2006). False positivity due to the presence of cross-reactive antibodies resulting from sequential or co-infection with flaviviruses in human clinical specimens limits the accuracy of currently used JE MAC-ELISA (Garg et al. 2012). The antigenic differences among JEV GI and GIII strains result in partial protection against GI strains in animals immunized with GIII strains (Ye et al. 2014). Hence, JE MAC-ELISA assays based on antigen derived from JEV GIII need to be re-evaluated for its sensitivity and specificity to diagnose the prevalent GI strains. All this prompted us to develop a JEV GI-specific IgM detection assay.
Flavivirus E protein is responsible for eliciting an antiviral immune response. Co-expression of the prM-E coding genes in mammalian cells gives rise to small, non-infectious, self-assembled VLPs, biologically and immunologically equivalent to the native virion (Konishi and Mason, 1993). Cloning the 390–2477 nt sequence amplified from JEV GI strain 0,945,054 in mammalian expression vector pcDNA 3.1 + resulted in expression and extracellular secretion of JEV E protein in the form of VLP (Fig. 7). Signal peptide located at the C-terminus of nucleocapsid plays a vital role in translocation and extracellular secretion of the E protein in its mature form as VLP (Konishi and Mason, 1993). Flavivirus VLPs expressed in mammalian cells are gaining increasing importance in research on host-virus interactions, diagnostics, and vaccine development as the similar immunogenicity of the expressed protein in VLPs form with the native virus is established (Krol et al. 2019). The transient expression of JEV E protein in 40% of cells was achieved by transfection of the recombinant vector (pcDNA 3.1-JE-Sig-prM-E) in BHK-21 cells, which are widely used for the virus propagation and expression of higher levels of flaviviral proteins (Cruz et al. 1998). Cellular clones stably expressing the recombinant protein were obtained by single-cell purification and propagation under G418 (Fig. 2b). The expressed JEV E protein morphologically and immunologically mimicked the parental virus as detected by EM analysis, IFA, antigen capture ELISA, and Western blot using anti-JEV monoclonal and polyclonal antibodies. The long-term stability of the BHK-IE6 clone was established by detecting a good amount of VLPs in cell supernatant up to 40 passages. Considering all characteristics exhibited by the expressed VLPs, they can be explored as a vaccine as described earlier (Chang et al. 2020; Fan et al. 2018).
Induction by sodium butyrate increases the recombinant protein expression by BHK-IE6 clones, which carry the gene of interest under the control of the CMV promoter present in the expression vector. The amount of JEV E protein (35–37 µg/ml) expressed by the BHK-IE6 after induction by 28 mM of sodium butyrate is significantly higher than that of the F, JE-4B, and J12#26 cells reported earlier (Konishi et al., 2001; Kojima et al. 2003). Higher levels of recombinant protein expression exert a metabolic burden leading to cell toxicity and growth retardation (Wu et al. 2016). However, we did not detect cellular toxicity as the cell morphology, and growth characteristics were comparable to the control BHK-21 cells (Fig. 3). On the contrary, the expression of JEV E protein in Vero cells was non-productive, probably due to the cell toxicity induced by expressed proteins (Kojima et al. 2003). Upon single-cell dilution of the cells obtained by transfection, we obtained 39 G418-resistant clones, but only 20 of these were positive by IFA, indicating the low frequency of recombination of the insert downstream to translation initiation elements. Extracellular secretion of expressed E protein also suggests that the intact genomic insert is located downstream of the translation initiation element under the transcription control of the CMV promoter.
Improvement of the currently available JE IgM ELISA using the purified immunodominant E protein as an antigen reduces the cross-reactivity (Innis et al. 1989; Lewthwaite et al. 2010). Our data indicate that JEV GI VLPs generated in this study can replace the JEV GIII whole virus as a superior antigen in IgM ELISA because both the ELISA, newly developed and conventional ELISA, correctly established the identity of JE IgM positive and negative sera and gave 100% sensitivity, and no cross-reactivity in sera from dengue cases was observed in newly developed ELISA and gave 100% specificity of the assay.
The 8 of the 10 JE and DEN dual IgM positive sera confirmed by conventional assays turned negative by the newly developed JE E antigen-based IgM ELISA. The 2 positive sera giving borderline positive OD values tested positive in both the dengue IgM ELISA and newly developed JE E antigen-based ELISA. Similarly, we obtained negative results for 9/10 sera tested indeterminate by the conventional JE IgM ELISA. These results might be due to exposure to both the JEV and DNEV, or the false-positive IgM ELISA results are frequently associated with autoimmune diseases, leptospirosis, and rheumatoid arthritis factor (Henle et al. 1979; Berlioz-Arthaud & Gurusamy, 2008). Negative JE IgM ELISA results obtained with all of the dengue IgM positive sera with newly developed JE E antigen-based IgM ELISA suggests it is not cross-reacting with the anti-DENV IgM and has increased specificity towards anti-JEV IgM.
In conclusion, JE is an important cause of epidemic encephalitis, so the availability of sensitive, specific, and rapid diagnostics assay is necessary to estimate the disease burden and to devise control strategies. Our study indicates that replacing cell culture-derived JEV GIII whole virus antigen with expressed VLP from JEV GI will yield more specific results in cases where multiple flaviviruses are co-circulating. All these are preliminary results; However, before implementing it in routine diagnostics, it must be passed through testing of sera collected from laboratory-confirmed non-JE AES cases and population in the JE endemic regions, which becomes a major limitation of this study.
References
Ali A, Igarashi A (1997) Antigenic and genetic variations among Japanese encephalitis virus strains belonging to genotype 1. Microbiol Immunol 41:241–252
Alonso Villela SM, Kraïem H, Bouhaouala-Zahar B, Bideaux C, Aceves Lara CA, Fillaudeau L (2020) A protocol for recombinant protein quantification by densitometry. Microbiologyopen 9:1175–1182. https://doi.org/10.1002/mbo3.1027
Berlioz-Arthaud A, Gurusamy A (2008) Comparison of PanBio dengue IgM ELISA assay with pentax dengue IgM particle agglutination assay to evaluate factors affecting false positive results. Southeast Asian J Trop Med Public Health 39:55–61
Cecilia D, Gadkari DA, Kedarnath N, Ghosh SN (1988) Epitope mapping of Japanese encephalitis virus envelope protein using monoclonal antibodies against an Indian strain. J Gen Virol 69:2741–2747. https://doi.org/10.1099/0022-1317-69-11-2741
Centers for Disease Control and Prevention (CDC) (2013) Investigational drug available directly from CDC for the treatment of infections with free-living amebae.vol. 62.
Chang H, Chiao J, Hsu L, Lin C, Wu L, Shu Y, Chang F, Chang H, Kuo C (2020) Mosquito cell-derived Japanese encephalitis virus-like particles induce specific humoral and cellular immune responses in mice. Viruses 12(3):336. https://doi.org/10.3390/v12030336
Cruz HJ, Moreira JL, Stacey G, Dias EM, Hayes K, Looby D, Griffiths B, Carrondo MJT (1998) Adaptation of BHK cells producing a recombinant protein to serum-free media and protein-free medium. Cytotechnology 26:59–64. https://doi.org/10.1023/A:1007951813755
Cuzzubbo AJ, Endy TP, Vaughn DW, Solomon T, Nisalak A, Kalayanarooj S, Dung NM, Warrilow D, Aaskov J, Devine PL (1999) Evaluation of a new commercially available immunoglobulin M capture enzyme-linked immunosorbent assay for diagnosis of Japanese encephalitis infections. J Clin Microbiol 12:3738–3741. https://doi.org/10.1128/JCM.37.11.3738-3741.1999
Fan Y, Chen J, Lin W, Chen Y, Wu H, Su H, Chiou T, Wu R, Yin H, Liao W, Chang J, Chiou S (2018) Genotype I of Japanese encephalitis virus virus-like particles elicit sterilizing immunity against genotype I and III viral challenge in swine. Sci Rep 8(1):7481. https://doi.org/10.1038/s41598-018-25596-1
Gadkari DA, Shaikh BH (1984) IgM antibody capture ELISA in the diagnosis of Japanese encephalitis, West Nile & dengue virus infections. Indian J Med Res 80:613–619
Gangodkar S, Jain P, Dixit N, Ghosh K, Basu A (2010) Dengue virus-induced autophagosomes and changes in endomembrane ultrastructure imaged by electron tomography and whole-mount grid-cell culture techniques. J Electron Microsc (tokyo) 59:503–511. https://doi.org/10.1093/jmicro/dfq063
Gangwar RS, Shil P, Cherian SS, Gore MM (2011) Delineation of an epitope on domain I of Japanese encephalitis virus envelope glycoprotein using monoclonal antibodies. Virus Res 158:179–187. https://doi.org/10.1016/j.virusres.2011.03.030
Garg RK, Malhotra HS, Gupta A, Kumar N, Jain A (2012) Concurrent dengue virus and Japanese encephalitis virus infection of the brain: is it co-infection or co-detection? Infection 40:589–593. https://doi.org/10.1007/s15010-012-0284-z
Henle G, Lennette ET, Alspaugh MA, Henle W (1979) Rheumatoid factor as a cause of positive reactions in tests for Epstein-Barr virus-specific IgM antibodies. Clin Exp Immunol 36:415–422
Innis BL, Nisalak A, Nimmannitya S, Kusalerdchariya S, Chongswasdi V, Suntayakorn S, Puttisri P, Hoke CH (1989) An enzyme-linked immunosorbent assay to characterize dengue infections where dengue and Japanese encephalitis co-circulate. Am J Trop Med Hyg 4:418–427. https://doi.org/10.4269/ajtmh.1989.40.418
Johnson BW, Goodman CH, Jee Y, Featherstone DA (2016) Differential diagnosis of Japanese encephalitis virus infections with the inbios JE Detect™ and DEN Detect™ MAC-ELISA kits. Am J Trop Med Hyg 94:820–828. https://doi.org/10.4269/ajtmh.15-0631
Kedarnath N, Dayaraj C, Sathe PS, Gadkari DA, Dandawate CN, Goverdhan MK, Ghosh SN (1986) Monoclonal antibodies against Japanese encephalitis virus. Indian J Med Res 84:125–133
Kojima A, Yasuda A, Asanuma H, Ishikawa T, Takamizawa A, Yasui K, Kurata T (2003) Stable high-producer cell clone expressing virus-like particles of the Japanese encephalitis virus E protein for a second-generation subunit vaccine. J Virol 77:8745–8755. https://doi.org/10.1128/jvi.77.16.8745-8755.2003
Konishi E, Fujii A, Mason PW (2001) Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J Virol 75:2204–12. https://doi.org/10.1128/jvi.75.5.2204-2212.2001
Konishi E, Mason PW (1993) Proper maturation of the Japanese encephalitis virus envelope glycoprotein requires cosynthesis with the premembrane protein. J Virol 67:1672–1675. https://doi.org/10.1128/jvi.67.3.1672-1675.1993
Krol E, Brzuska G, Szewczyk B (2019) Production and biomedical application of flavivirus-like particles. Trends Biotechnol 37:1202–1216. https://doi.org/10.1016/j.tibtech.2019.03.013
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685. https://doi.org/10.1038/227680a0
Lewthwaite P, Veera Shankar M, Tio PH, Daly J, Last A, Ravikumar R, Desai A, Ravi V, Cardosa JM, Solomon T (2010) Evaluation of two commercially available ELISAs for the diagnosis of Japanese encephalitis applied to field samples. Trop Med Int Heal 15:811–818. https://doi.org/10.1111/j.1365-3156.2010.02537.x
Mansfield KL, Hernández-Triana LM, Banyard AC, Fooks AR, Johnson N (2017) Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol 201:85–92. https://doi.org/10.1016/j.vetmic.2017.01.014
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. 1st edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT (2000) Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 38:1823–1826. https://doi.org/10.1128/jcm.38.5.1823-1826.2000
Phelan K, May KM (2016) Basic techniques in mammalian cell tissue culture. Curr Protoc Toxicol;2016:A.3B.1-A.3B.22. https://doi.org/10.1002/cptx.13.
Ravi V, Desai A, Balaji M, Apte MP, Lakshman L, Subbakrishna DK, Sridharan G, Dhole TN, Ravikumar BV (2006) Development and evaluation of a rapid IgM capture ELISA (JEV-Chex) for the diagnosis of Japanese encephalitis. J Clin Virol 35:429–434. https://doi.org/10.1016/j.jcv.2005.11.004
Sarkar A, Taraphdar D, Mukhopadhyay SK, Chakrabarti S, Chatterjee S (2012) Molecular evidence for the occurrence of Japanese encephalitis virus genotype I and III infection associated with acute Encephalitis in Patients of West Bengal, India, 2010. Virol J 9:271. https://doi.org/10.1186/1743-422X-9-271
Schuh AJ, Ward MJ, Leigh Brown AJ, Barrett ADT (2014) Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. J Virol 88:4522–4532. https://doi.org/10.1128/JVI.02686-13
Taylor TJ, Diaz F, Colgrove RC, Bernard KA, DeLuca NA, Whelan SPJ, Knipe DM (2016) Production of immunogenic West Nile virus-like particles using a herpes simplex virus 1 recombinant vector. Virology 496:186–193. https://doi.org/10.1016/j.virol.2016.06.006
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 76(9):4350–4354. https://doi.org/10.1073/pnas.76.9.4350
Uchil PD, Satchidanandam V (2001) Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am J Trop Med Hyg 65:242–251. https://doi.org/10.4269/ajtmh.2001.65.242
WHO (2006) Global Advisory Committee on Vaccine Safety, 29–30 November. Wkly Epidemiol Rec 2007(82):18–24
World Health Organization (2015) Japanese encephalitis. Who 2015:47–66
Wu G, Yan Q, Jones J, Tang Y, Fong S, Koffas M (2016) Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol 34(8):652–664. https://doi.org/10.1016/j.tibtech.2016.02.010
Ye Q, Li X, Zhao H, Deng Y, Xu Y, Wang H, Liang G, Qin C (2014) Reduction of neutralization antibody against heterologous circulating strains in adults immunized with Japanese encephalitis live vaccine. Human Vaccines & Immunotherapeutics 10(9):2704–2705. https://doi.org/10.4161/hv.29509
Acknowledgements
The authors are thankful to Mrs. Shubhangi Mahamuni (STO) and Mrs. Daya Pavitrakar (TO) for their technical help in this work. Provision of standard serum panel and technical guidance was offered by Dr. Paresh Shah, Scientist E and Group Leader, Diagnostic Research Facility, ICMR-NIV, Pune. Authors are thankful to Dr. Atanu Basu, Scientist G, ICMR-NIV, Pune, for supporting the electron microscopic analysis. Consistent encouragement and support by Prof. Priya Abraham, Director, ICMR-NIV, Pune, are highly appreciated. Support from CSIR-UGC and Savitribai Phule Pune University for the research fellowship and registration of DN Mali is appreciated.
Funding
This work was financially supported by the Indian Council Medical Research, New Delhi, under the project ENC1603 to the corresponding author.
Author information
Authors and Affiliations
Contributions
VPB conceived the concept and funds, supervised the work, and validated the results. DNM conducted all the experiments, analyzed the data, and wrote the original draft of manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Consent to participate
Not applicable.
Consent for publication
All authors approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mali, D., Bondre, V. Japanese encephalitis genotype I virus-like particles stably expressed in BHK-21 cells serves as potential antigen in JE IgM ELISA. Appl Microbiol Biotechnol 106, 1945–1955 (2022). https://doi.org/10.1007/s00253-022-11825-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11825-1