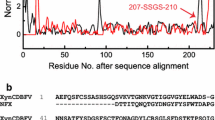
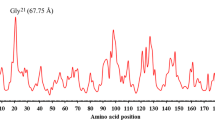
Abstract
A hyperthermostable xylanase XYN10B from Thermotoga maritima (PDB code 1VBR, GenBank accession number KR078269) was subjected to site-directed and error-prone PCR mutagenesis. From the selected five mutants, the two site-directed mutants (F806H and F806V) showed a 3.3–3.5-fold improved enzyme half-life at 100 °C. The mutant XYNA generated by error-prone PCR showed slightly improved stability at 100 °C and a lower Km. In XYNB and XYNC, the additional mutations over XYNA decreased the thermostability and temperature optimum, while elevating the Km. In XYNC, two large side-chains were introduced into the protein’s interior. Micro-differential scanning calorimetry (DSC) showed that the melting temperature (Tm) dropped in XYNB and XYNC from 104.9 °C to 93.7 °C and 78.6 °C, respectively. The detrimental mutations showed that extremely thermostable enzymes can tolerate quite radical mutations in the protein’s interior and still retain high thermostability. The analysis of mutations (F806H and F806V) in a hydrophobic area lining the substrate-binding region indicated that active site hydrophobicity is important for high activity at extreme temperatures. Although polar His at 806 provided higher stability, the hydrophobic Phe at 806 provided higher activity than His. This study generates an understanding of how extreme thermostability and high activity are formed in GH10 xylanases.
Key points
• Characterization and molecular dynamics simulations of TmXYN10B and its mutants
• Explanation of structural stability of GH10 xylanase






Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Basit A, Liu J, Rahim K, Jiang W, Lou H (2018) Thermophilic xylanases: from bench to bottle. Crit Rev Biotechnol 38:989–1002
Bhalla A, Bansal N, Kumar S, Bischoff KM, Sani RK (2013) Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour Technol 128:751–759
Bommarius AS, Paye MF (2013) Stabilizing biocatalysts. Chem Soc Rev 42:6534–6565
Camilloni C, Bonetti D, Morrone A, Giri R, Dobson CM, Brunori M, Gianni S, Vendruscolo M (2016) Towards a structural biology of the hydrophobic effect in protein folding. Sci Rep 6:28285
Chaudhary R, Kuthiala T, Singh G, Rarotra S, Kaur A, Arya SK, Kumar P (2021) Current status of xylanase for biofuel production: a review on classification and characterization. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01948-2
Chawachart N, Anbarasan S, Turunen S, Li H, Khanongnuch C, Hummel M, Sixta H, Granstrom T, Lumyong S, Turunen O (2014) Thermal behaviour and tolerance to ionic liquid [emim]OAc in GH10 xylanase from Thermoascus aurantiacus SL16W. Extremophiles 18:1023–1034
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29:3–23
Denisov VP, Schlessman JL, Garcia-Moreno EB, Halle B (2004) Stabilization of internal charges in a protein: water penetration or conformational change? Biophys J 87:3982–3994
Dougherty RC (1998) Temperature and pressure dependence of hydrogen bond strength: a perturbation molecular orbital approach. J Chem Phys 109:7372–7378
Dumon C, Varvak A, Wall MA, Flint JE, Lewis RJ, Lakey JH, Morland C, Luginbuhl P, Healey S, Todaro T, DeSantis G, Sun M, Parra-Gessert L, Tan X, Weiner DP, Gilbert HJ (2008) Engineering hyperthermostability into a GH11 xylanase is mediated by subtle changes to protein structure. J Biol Chem 283:22557–22564
Ericsson UB, Hallberg BM, Detitta GT, Dekker N, Nordlund P (2006) Thermofluor-based high-throughput stability optimization of proteins for structural studies. Anal Biochem 357:289–298
Gromiha MM, Pathak MC, Saraboji K, Ortlund EA, Gaucher EA (2013) Hydrophobic environment is a key factor for the stability of thermophilic proteins. Proteins 81:715–721
Hämäläinen J, Granström T, Mollerup F, Wang Y, Xiong H, Turunen O (2016) Effect of enzymatic high temperature prehydrolysis on the subsequent cellulose hydrolysis of steam-pretreated spruce in high solids concentration. J Chem Technol Biotechnol 91:1844–1852
Hebal H, Parviainen A, Anbarasan S, Li H, Makkonen L, Bankar S, King AWT, Kilpeläinen I, Benallaoua S, Turunen O (2020) Inhibition of hyperthermostable xylanases by superbase ionic liquids. Process Biochem 95:148–156
Hebal H, Boucherba N, Binay B, Turunen O (2021) Activity and stability of hyperthermostable cellulases and xylanases in ionic liquids. Biocatal Biotransformation 39:242–259
Ihsanawati KT, Kaneko T, Morokuma C, Yatsunami R, Sato T, Nakamura S, Tanaka N (2005) Structural basis of the substrate subsite and the highly thermal stability of xylanase 10B from Thermotoga maritima MSB8. Proteins 61:999–1009
Johnson RJ, Savas CJ, Kartje Z, Hoops GC (2014) Rapid and adaptable measurement of protein thermal stability by differential scanning fluorimetry: updating a common biochemical laboratory experiment. J Chem Educ 91:1077–1080
Kamerzell TJ, Middaugh CR (2008) The complex inter-relationships between protein flexibility and stability. J Pharm Sci 97:3494–3517
Kashif A, Tran LH, Jang SH, Lee CW (2017) Roles of active-site aromatic residues in cold adaptation of Sphingomonas glacialis esterase EstSP1. ACS Omega 2:8760–8769
Katewadee B, Penchit C, Pattanop K, Verawat C (2021) Enhancement of catalytic performance of a metagenome-derived thermophilic oligosaccharide-specific xylanase by binding module removal and random mutagenesis. J Biosci Bioeng 131:13–19
Kumar V, Dangi AK, Shukla P (2018) Engineering thermostable microbial xylanases toward its industrial applications. Mol Biotechnol 60:226–235
Lai Z, Zhou C, Ma X, Xue Y, Ma Y (2021) Enzymatic characterization of a novel thermostable and alkaline tolerant GH10 xylanase and activity improvement by multiple rational mutagenesis strategies. Int J Biol Macromol 170:164–177
Li H, Kankaanpää A, Xiong H, Hummel M, Sixta H, Ojamo H, Turunen O (2013) Thermostabilization of extremophilic Dictyoglomus thermophilum GH11 xylanase by an N-terminal disulfide bridge and the effect of ionic liquid [emim]OAc on the enzymatic performance. Enzym Microb Technol 53:414–419
Li G, Fang X, Su F, Chen Y, Xu L, Yan Y (2018) Enhancing the thermostability of Rhizomucor miehei lipase with a limited screening library by rational-design point mutations and disulfide bonds. Appl Environ Microbiol 84:e02129–e02117
Li G, Zhou X, Li Z, Liu Y, Liu D, Miao Y, Wan Q, Zhang R (2021) Significantly improving the thermostability of a hyperthermophilic GH10 family xylanase XynAF1 by semi-rational design. Appl Microbiol Biotechnol 105:4561–4576
Lim SJ, Oslan SN (2021) Native to designed: microbial α-amylases for industrial applications. PeerJ 9:e11315
Miyazaki K, Takenouchi M, Kondo H, Noro N, Suzuki M, Tsuda S (2006) Thermal stabilization of Bacillus subtilis family-11 xylanase by directed evolution. J Biol Chem 281:10236–10242
Nakamura A, Tsukada T, Auer S, Furuta T, Wada M, Koivula A, Igarashi K, Samejima M (2013) The tryptophan residue at the active site tunnel entrance of Trichoderma reesei cellobiohydrolase Cel7A is important for initiation of degradation of crystalline cellulose. J Biol Chem 288:13503–13510
Prajapati AS, Pawar VA, Panchal KJ, Sudhir AP, Dave BR, Patel DH, Subramanian RB (2018) Effects of substrate binding site residue substitutions of xynA from Bacillus amyloliquefaciens on substrate specificity. BMC Biotechnol 18:9
Shoichet BK, Baase WA, Kuroki R, Matthews BW (1995) A relationship between protein stability and protein function. Proc Natl Acad Sci U S A 92:452–456
Simpson HD, Haufler UR, Daniel RM (1991) An extremely thermostable xylanase from the thermophilic eubacterium Thermotoga. Biochem J 277:413–417
Song L, Tsang A, Sylvestre M (2015) Engineering a thermostable fungal GH10 xylanase, importance of N-terminal amino acids. Biotechnol Bioeng 112:1081–1091
Taylor MP, Eley KL, Martin S, Tuffin M, Burton SG, Cowan DA (2009) Thermophilic ethanologenesis: future prospects for second-generation bioethanol production. Trends Biotechnol 27:398–405
Tian L, Liu S, Wang S, Wang L (2016) Ligand-binding specificity and promiscuity of the main lignocellulolytic enzyme families as revealed by active-site architecture analysis. Sci Rep 6:23605
Torktaz I, Karkhane AA, Hemmat J (2018) Rational engineering of Cel5E from Clostridium thermocellum to improve its thermal stability and catalytic activity. Appl Microbiol Biotechnol 102:8389–8402
Tu T, Luo H, Meng K, Cheng Y, Ma R, Shi P, Huang H, Bai Y, Wang Y, Zhang L, Yao B (2015) Improvement in thermostability of an Achaetomium sp. strain Xz8 endopolygalacturonase via the optimization of charge-charge interactions. Appl Environ Microbiol 81:6938–6944
Tu T, Li Y, Luo Y, Wang Z, Wang Y, Luo H, Yao B (2018) A key residue for the substrate affinity enhancement of a thermophilic endo-polygalacturonase revealed by computational design. Appl Microbiol Biotechnol 102:4457–4466
Turunen O, Jänis J, Fenel F, Leisola M (2004) Engineering the thermotolerance and pH optimum of family 11 xylanases by site-directed mutagenesis. Methods Enzymol 388:156–167
van Dijk E, Hoogeveen A, Abeln S (2015) The hydrophobic temperature dependence of amino acids directly calculated from protein structures. PLoS Comput Biol 11:e1004277
Wang Y, Fu Z, Huang H, Zhang H, Yao B, Xiong H, Turunen O (2012) Improved thermal performance of Thermomyces lanuginosus GH-11 xylanase by engineering of an N-terminal disulfide bridge. Bioresour Technol 112:275–279
Wang K, Luo H, Tian J, Turunen O, Huang H, Shi P, Hua H, Wang C, Wang S, Yao B (2014) Thermostablility improvement of a Streptomyces xylanase by introducing proline and glutamic acid residues. Appl Environ Microbiol 80:2158–2165
Wang Y, Bai Y, Shu T, Fan P, Zhang H, Turunen O, Xiong H, Yu L (2020) Characterization of a versatile glycoside hydrolase Cel5M from Pectobacterium carotovorum HG-49 for ramie degumming. Text Res J 90:1602–1615
Winterhalter C, Liebl W (1995) Two extremely thermostable xylanases of the hyperthermophilic bacterium Thermotoga maritima MSB8. Appl Environ Microbiol 61:1810–1815
Wu Z, Zhao C, Huang Y, Ye F, Zhao G (2020) Molecular mechanism underlying the effects of temperature and pH on the size and surface charge of octenylsuccinated oat β-glucan aggregates. Carbohydr Polym 237:116115
Yang J, Han Z (2018) Understanding the positional binding and substrate interaction of a highly thermostable GH10 xylanase from Thermotoga maritima by molecular docking. Biomolecules 8:64
Yang J, Ma T, Shang-guan F, Han Z (2020) Improving the catalytic activity of thermostable xylanase from Thermotoga maritima via mutagenesis of non-catalytic residues at glycone subsites. Enzym Microb Technol 139:109579
Yi Y, Xu S, Kovalevsky A, Zhang X, Liu D, Wan Q (2021) Characterization and structural analysis of a thermophilic GH11 xylanase from compost metatranscriptome. Appl Microbiol Biotechnol 105:7757–7767
You C, Huang Q, Xue H, Xu Y, Lu H (2010) Potential hydrophobic interaction between two cysteines in interior hydrophobic region improves thermostability of a family 11 xylanase from Neocallimastix patriciarum. Biotechnol Bioeng 105:861–870
Yu T, Anbarasan S, Wang Y, Telli K, Aslan AS, Su Z, Zhou Y, Zhang L, Iivonen P, Havukainen S, Mentunen T, Hummel M, Sixta H, Binay B, Turunen O, Xiong H (2016) Hyperthermostable Thermotoga maritima xylanase XYN10B shows high activity at high temperatures in the presence of biomass-dissolving hydrophilic ionic liquids. Extremophiles 20:515–524
Acknowledgements
This work was financially supported by the project (no. DY135-B2-07) from the China Ocean Mineral Resources R&D Association, Hubei Provincial Technical Innovation Program (no. 2018ABA093).
Author information
Authors and Affiliations
Contributions
YW, OT, and HX conceived and designed research. YW, JW, and ZZ conducted experiments. JY and OT analyzed data. YW, OT, and HX wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, Y., Wang, J., Zhang, Z. et al. High-temperature behavior of hyperthermostable Thermotoga maritima xylanase XYN10B after designed and evolved mutations. Appl Microbiol Biotechnol 106, 2017–2027 (2022). https://doi.org/10.1007/s00253-022-11823-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-022-11823-3