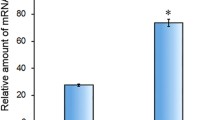
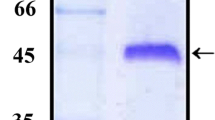
Abstract
Vibrio parahaemolyticus is one of the major pathogens responsible for vibriosis and zoonotic infections in teleosts, marine invertebrates, and also humans through consumption of contaminated or unprocessed seafood. Emergence of resistance against current accessible antibiotics and spread to the food chain and environment necessitate the development of safe and effective subunit vaccine against this bacterium. Many bacteria including V. parahaemolyticus produce extracellular curli fibrils, heteropolymeric filaments of major and minor subunit, which have been implicated in adhesion, biofilm formation, and virulence. Adhesins are the primary contact points with the host which help in establishing infection and colonization. CsgA, an adhesin, is the major structural component of the curli fiber that forms homopolymers of several hundred units. Due to their exposure on the cell surface, the curli fibers are recognized by the host’s immune system, would generate high immune response, and therefore can serve as effective vaccine candidate. In the present study, we describe characterization of the csgA gene, and preparation of recombinant soluble CsgA of V. parahaemolyticus (rVpCsgA), and evaluation of its vaccine potential. Immunization of BALB/c mice with the rVpCsgA mounted a strong immune response. Cellular immune assays such as antibody isotyping, in vitro splenocyte proliferation analysis, and cytokine profiling revealed mixed T-helper cell immune response. The anti-rVpCsgA antiserum was agglutination positive and specifically cross-reacted with the curli CsgA present on the outer membrane of V. parahaemolyticus cells, thus demonstrating its neutralization potential. One hundred percent survival of the immunized mice upon challenge with the lethal dosage of the bacterium established that the rVpCsgA could serve as an effective vaccine against the bacterium.
Key points
• Recombinant histidine-tagged CsgA of V. parahaemolyticus, rVpCsgA, was purified.
• The rVpCsgA immunization produced mixed immune response and agglutinating antibodies.
• Immunization with the rVpCsgA protected mice against V. parahaemolyticus challenge.










Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its supplementary information files.
References
Adamson A, Ghoreschi K, Rittler M, Chen Q, Sun H-W, Vahedi G, Kanno Y, Stetier-Stevenson WG, O’Shea JJ, Laurence A (2013) Tissue inhibitor of metalloproteinase 1 is preferentially expressed in Th1 and Th17 T-helper cell subsets and is a direct stat target gene. PLoS ONE 8:e59367. https://doi.org/10.1371/journal.pone.0059367
Amati AL, Zakrzewicz A, Siebers K, Wilker S, Heldmann S, Zakrzewicz D, Hecker A, McIntosh JM, Padberg W, Grau V (2017) Chemokines (CCL3, CCL4, and CCL5) inhibit ATP-induced release of IL-1β by monocytic cells. Mediat Inflamm 2017:1434872. https://doi.org/10.1155/2017/1434872
Bhowmick R, Ghosal A, Das B, Koley H, Saha DR, Ganguly S, Nandy RK, Bhadra RK, Chatterjee NS (2008) Intestinal adherence of Vibrio cholerae involves a coordinated interaction between colonization factor GbpA and mucin. Infect Immun 76:4968–4977. https://doi.org/10.1128/IAI.01615-07
Bielinska AU, O’Konek JJ, Janczak KW, Baker JR Jr (2016) Immunomodulation of TH2 biased immunity with mucosal administration of nanoemulsion adjuvant. Vaccine 34:4017–4024. https://doi.org/10.1016/j.vaccine.2016.06.043
Bravo S, Midtlyng PJ (2007) The use of fish vaccines in the Chilean salmon industry 1999-2003. Aquaculture 270:36–42
Carson WF, Kunkel SF (2017) Type I and II cytokine superfamilies in inflammatory responses. In: Cavaillon JM, Singer M (eds) Inflammation: from molecular and cellular mechanisms to the clinic. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, pp 587–618. https://doi.org/10.1002/9783527692156.ch24
CDC (2019) Centers for disease control and prevention, national center for emerging and zoonotic infectious diseases (NCEZID), division of foodborne, waterborne, and environmental diseases. Vibrio species causing vibriosis. https://www.cdc.gov/vibrio/faq.html/
Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammer M, Nomark S, Hultgren S (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851–855. https://doi.org/10.1126/science.1067484
Chatrath S, Gupta VK, Garg LC (2014) The PGRS domain is responsible for translocation of PE_PGRS30 to cell poles while the PE and the C-terminal domains localize it to the cell wall. FEBS Letters 588:990–994. https://doi.org/10.1016/j.febslet.2014.01.059
Dueholm MS, Albertsen M, Otzen D, Nielsen PH (2012) Curli functional amyloid systems are phylogenetically widespread and display large diversity in operon and protein structure. PLoS One 7:e51274. https://doi.org/10.1371/journal.pone.0051274
Elahi S, Holmstrom J, Gerdts V (2007) The benefits of using diverse animal models for studying pertussis. Trends Microbiol 15:462–468. https://doi.org/10.1016/j.tim.2007.09.003
Fujino T, Okuno Y, Nakada D, Aoyama A, Mukai T, Ueho T (1953) On the bacteriological examination of shirasu food poisoning. Med J Osaka Univ 4:299–304
Gioia CAC, de Sousa AB, Cruz SC, Junior FCS, Andrade AFB, Sassi RM, Frasch CE, Milagres LG (2005) Effect of a booster dose of serogroup B meningococcal vaccine on antibody response to Neisseria meningitidis in mice vaccinated with different immunization schedules. FEMS Immunol Med Microbiol 44:35–42. https://doi.org/10.1016/j.femsim.2004.11.013
Hauge S, Madhun A, Cox RJ, Haaheim LR (2007) Quality and kinetics of the antibody response in mice after three different low-dose influenza virus vaccination strategies. Clin Vaccine Immunol 14:978–983. https://doi.org/10.1128/CVI.00033-07
Kan A, Birnbaum DP, Praveschotinunt P, Joshi NS (2019) Congo red fluorescence for rapid in situ characterization of synthetic curli systems. Appl Environ Microbiol 85:e00434–e00419. https://doi.org/10.1128/AEM.00434-19
Karan S, Dash P, Kaushik H, Sahoo PK, Garg LC, Dixit A (2016) Structural and functional characterization of recombinant interleukin-10 from Indian major carp Labeo rohita. J Immunol Res 2016:3962596–3962511. https://doi.org/10.1155/2016/3962596
Karan S, Mohapatra A, Sahoo PK, Garg LC, Dixit A (2020) Structural-functional characterization of recombinant apolipoprotein A-I from Labeo rohita demonstrates heat-resistant antimicrobial activity. Appl Microbiol Biotechnol 104:145–159. https://doi.org/10.1007/s00253-019-10204-7
Khalouie F, Mousavi SL, Nazarian S, Amani J, Pourfarzam P (2017) Immunogenic evaluation of chimeric recombinant protein against ETEC, EHEC and Shigella. Mol Biol Res Commun 6:101-112. doi: https://doi.org/10.22099/mbrc.2017.4081
Kim MN, Bang HJ (2008) Detection of marine pathogenic bacterial Vibrio species by multiplex polymerase chain reaction (PCR). J Environ Biol 29:543–546
Kindt TJ, Goldsby RA, Osborne BA, Kuby J (2007) The major histocompatibility complex and antigen presentation. In: Ahr K (ed) Kuby immunology, 6th edn. WH Freeman and Company, New York, pp 189–244
Kiros TG, Levast B, Auray G, Strom S, van Kessel J, Gerdts V (2012) The importance of animal models in the development of vaccines. In: Baschieri S (ed) Innovation in vaccinology. Springer, Dordrecht, pp 251–264. https://doi.org/10.1007/978-94-007-4543-8_11
Kolls JK, McCray PB Jr, Chan YR (2008) Cytokine-mediated regulation of antimicrobial proteins. Nat Rev Immunol 8:829–835. https://doi.org/10.1030/nri2433
Langermann S, Mollby R, Burlein JE, Palaszynski SR, Auguste CG, DeFuscoA Strouse R, Schenerman MA, Hultgren SJ, Pinkner JS, Winberg J, Guldevall L, Soderhall M, Ishikawa K, Normark S, Koenig S (2000) Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J Infect Dis 181:774–778. https://doi.org/10.1086/315258
Lebre MC, Burwell T, Vieira PL, Lora J, Coyle AJ, Kapsenberg ML, Clausen BE, De Jong EC (2005) Differential expression of inflammatory chemokines by Th1- and Th2-cell promoting dendritic cells: a role for different mature dendritic cell populations in attracting appropriate effector cells to peripheral sites of inflammation. Immunol Cell Biol 83:525–535. https://doi.org/10.1111/j.1440-1711.2005.01365.x
Letchumanan V, Yin WF, Lee LH, Chan KG (2015) Prevalence and antimicrobial susceptibility of Vibrio parahaemolyticus isolated from retail shrimps in Malaysia. Front Microbiol 6:33. https://doi.org/10.3389/fmicb.2015.00033
Li C, Ye Z, Wen L, Chen R, Tian L, Zhao F, Pan J (2014) Identification of a novel vaccine candidate by immunogenic screening of Vibrio parahaemolyticus outer membrane proteins. Vaccine 32:6115–6121. https://doi.org/10.1016/j.vaccine.2014.08.077
Li H, Ye MZ, Peng B, Wu HK, Xu CX, Xiong XP, Wang C, Wang SY, Peng XX (2010) Immunoproteomic identification of polyvalent vaccine candidates from Vibrio parahaemolyticus outer membrane proteins. J Proteome Res 9:2573–2583. https://doi.org/10.1021/pr1000219
Liao W, Lin JX, Leonard WJ (2011) IL-2 family cytokines: new insights into the complex roles of IL-2 as a broad regulator of T helper cell differentiation. Curr Opin Immunol 23:598–604. https://doi.org/10.1016/j.coi.2011.08.003
Lin JHY, Chen TY, Chen MS, Chen HE, Chou RL, Chen TI, Su MS, Yang HL (2006) Vaccination with three inactivated pathogens of cobia (Rachycentron canadum) stimulates protective immunity. Aquaculture 255:125–132
Ling R, Logar-Henderson C, Fisman D (2019) El Nino southern oscillation predicts vibriosis risk in the United States. Int J Infect Dis 79:18. doi:https://doi.org/10.1016/j.ijid.2018.11.060
Logar-Henderson C, Ling R, Tuite AR, Fisman DN (2019) Effects of large-scale oceanic phenomena on non-cholera vibriosis incidence in the United States: implications for climate change. Epidemiol Infect 147:e243. https://doi.org/10.1017/S0950268819001316
Luna-Pineda VM, Moreno-Fierros L, Cázares-Domínguez V, Ilhuicatzi-Alvarado D, Ochoa SA, Cruz-Cordova A, Valencia-Mayoral P, Rodríguez-Leviz A, Xicohtencatl-Cortes J (2019) Curli of uropathogenic Escherichia coli enhance urinary tract colonization as a fitness factor. Front Microbiol 10:2063. https://doi.org/10.3389/fmicb.2019.02063
Maier B, Wong GCL (2015) How bacteria use type IV pili machinery on surfaces. Trends Microbiol 23:775–788. https://doi.org/10.1016/j.tim.2015.09.002
Mao Z, Yu L, You Z, Wei Y, Liu Y (2007) Cloning, expression and immunogenicity analysis of five outer membrane proteins of Vibrio parahaemolyticus zj2003. Fish Shellfish Immunol 23:567–575. https://doi.org/10.1016/j.fsi.2007.01.004
Molaee N, Mosayebi G, Amozande-Nobaveh A, Soleyman MR, Abtahi H (2017) Evaluation of the immune response against recombinant proteins (TcpA, TcpB, and FlaA) as a candidate subunit cholera vaccine. J Immunol Res 2017:2412747–2412748. https://doi.org/10.1155/2017/2412747
Peng B, Ye JZ, Han Y, Zeng L, Zhang JY, Li H (2016) Identification of polyvalent protective immunogens from outer membrane proteins in Vibrio parahaemolyticus to protect fish against bacterial infection. Fish Shellfish Immunol 54:204–210. https://doi.org/10.1016/j.fsi.2016.04.012
Powell A, Pope EC, Eddy FE, Roberts EC, Shields RJ, Francis MJ, Smith P, Topps S, Reid J, Rowley AF (2011) Enhanced immune defences in Pacific white shrimp (Litopenaeus vannamei) post-exposure to a Vibrio vaccine. J Invertebr Pathol 107:95–99. https://doi.org/10.1016/j.jip.2011.02.006
Qian R, Chu W, Mao Z, Zhang C, Wei Y, Yu L (2007) Expression, characterization and immunogenicity of a major outer membrane protein from Vibrio alginolyticus. Acta Biochim Biophys Sin 39:194–200. https://doi.org/10.1111/j.1745-7270.2007.00268.x
http://cshprotocols.cshlp.org/content/2006/1/pdb.rec701.full
Reed LJ, Muench H (1938) A simple method of estimating fifty per cent end points. Am J Hyg 27:493–497
Schaller TH, Batich KA, Suryadevara CM, Desai R, Sampson JH (2017) Chemokines as adjuvants for immunotherapy: implications for immune activation with CCL3. Expert Rev Clin Immunol 13:1049–1060. https://doi.org/10.1080/1744666X.2017.1384313
Scheu S, Ali S, Ruland C, Arolt V, Alferink J (2017) The C-C chemokines CCL17 and CCL22 and their receptor CCR4 in CNS autoimmunity. Int J Mol Sci 18:2306. https://doi.org/10.3390/ijms18112306
Schulz SM, Kohler G, Holscher C, Iwakura Y, Alber G (2008) IL-17A is produced by Th17, γδ T cells and other CD4− lymphocytes during infection with Salmonella enterica serovar enteritidis and has a mild effect in bacterial clearance. Int Immunol 20:1129–1138. https://doi.org/10.1093/intimm/dxn069
Sharma M, Dixit A (2016) Immune response characterization and vaccine potential of a recombinant chimera comprising B cell epitope of Aeromonas hydrophila of outer membrane protein C and LTB. Vaccine 34:6259–6266. https://doi.org/10.1016/j.vaccine.2016.10.064
Shi G, Cox AC, Vistica BP, Tan C, Wawrousek EF, Gery I (2008) Phenotype switching by inflammation-inducing polarized Th17 cells, but not by Th1 cells. J Immunol 181:7205–7213. https://doi.org/10.4049/jimmunol.181.10.7205
Shimohata T, Takahashi A (2010) Diarrhea induced by infection of Vibrio parahaemolyticus. J Med Investig 57:179–182. https://doi.org/10.2152/jmi.57.179
Spellberg B, Edwards JE (2001) Type1/Type2 immunity in infectious diseases. Clin Infect Dis 32:76–102. https://doi.org/10.1086/317537
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold spring Harb Perspect biol. 6:1-16. Doi:https://doi.org/10.1101/cshperspect.a016295
Van Gerven N, Klein RD, Hultgren SJ, Remaut H (2015) Bacterial amyloid formation: structural insights into curli biogenesis. Trends Microbiol 11:693–706. https://doi.org/10.1016/j.tim.2015.07.010
Van Gerven N, Van der Verren SE, Reiter DM, Remaut H (2018) The role of functional amyloids in bacterial virulence. J Mol Biol 430:3657–3684. https://doi.org/10.1016/j.jmb.2018.07.010
Wang R, Deng Y, Deng Q, Sun D, Fang Z, Sun L, Wang Y, Gooneratne R (2020) Vibrio parahaemolyticus infection in mice reduces protective gut microbiota, augmenting disease pathways. Front Microbiol 11:73. https://doi.org/10.3389/fmicb.2020.00073
Wang X, Hammer ND, Chapman MR (2008) The molecular basis of functional bacterial amyloid polymerization and nucleation. J Biol Chem 283:21530–21539
Wang C, Liu Y, Li H, Xu WJ, Zhang H, Peng XX (2012) Identification of plasma-responsive outer membrane proteins and their vaccine potential in Edwardsiella tarda using proteomic approach. J Proteome 75:1263–1375. https://doi.org/10.1016/j.jprot.2011.11.001
Wilson KC, Center DM, Cruikshank WW (2004) The effect of interleukin-16 and its precursor on T lymphocyte activation and growth. Growth Factors 22:97–104. https://doi.org/10.1080/08977190410001704679
WHO (2014) Antimicrobial resistance global report on surveillance. World Health Organization. http://www.who.int/drugresistance/documents/surveillancereport/en/
Wu Q, Martin RJ, Rino JG, Breed R, Torres RM, Chu HW (2007) IL-23-dependent IL-17 production is essential in neutrophil recruitment and activity in mouse lung defense against respiratory Mycoplasma pneumonia infection. Microbes Infect 9:78–86. https://doi.org/10.1016/j.micinf.2006.10.012
Wynn TA (2003) IL-13 effector functions. Annu Rev Immunol 21:425–456. https://doi.org/10.1146/annurev.immunol.21.120601.141142
Yadav SK, Sahoo PK, Dixit A (2014) Characterization of immune response elicited by the recombinant outer membrane protein OmpF of Aeromonas hydrophila, a potential vaccine candidate in murine model. Mol Biol Rep 41:1837–1848
Yadav SK, Meena JK, Sharma M, Dixit A (2016) Recombinant outer membrane protein C of Aeromonas hydrophila elicits mixed immune response and generates agglutinating antibodies. Immunol Res 64:1087–1099. https://doi.org/10.1007/s12026-016-8807-9
Zha Z, Li C, Li W, Ye Z, Pan J (2016) LptD is a promising vaccine antigen and potential immunotherapeutic target for protection against Vibrio species infection. Sci Rep 6:38577. https://doi.org/10.1038/srep38577
Zhang L, Orth K (2013) Virulence determinants for Vibrio parahaemolyticus infection. Curr Opin Microbiol 16:70–77. https://doi.org/10.1016/j.mib.2013.02.002
Acknowledgments
The Indian Council of Medical Research, New Delhi is acknowledged for research fellowship to SK. Prof. M. R. Chapman, Molecular, Cellular and Developmental Biology, University of Michigan, Ann Arbor, MI, USA is gratefully acknowledged for kindly providing with the E. coli C600 csg knockout strain, E. coli LSR12.
Funding
The present study has been carried out with the financial support from the Department of Science and Technology PURSE grant (SR/PURSE/Phase2/11(C) 2015) to the Jawaharlal Nehru University, New Delhi and the intramural funding from the JNU, New Delhi, India.
Author information
Authors and Affiliations
Contributions
AD, DC, and SK conceived and designed the study. SK performed the experiments and acquired the data. SK, DC, and AD analyzed and interpreted the data. SK, DC, and AD drafted and revised the manuscript. All authors approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The work presented in this manuscript does not contain any study comprising human participants. The use of animals (BALB/c mice) for immunization was approved by the Institutional Animal Ethics Committee (IAEC) of the University (IAEC project code 08/2017), New Delhi. The guidelines prescribed by the IAEC were followed for the use of animals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 2.06 mb)
Rights and permissions
About this article
Cite this article
Karan, S., Choudhury, D. & Dixit, A. Immunogenic characterization and protective efficacy of recombinant CsgA, major subunit of curli fibers, against Vibrio parahaemolyticus. Appl Microbiol Biotechnol 105, 599–616 (2021). https://doi.org/10.1007/s00253-020-11038-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-11038-4