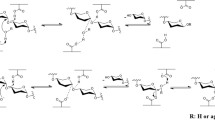
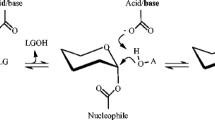
Abstract
The reversible hydrolytic property of glycosyl hydrolases (GHs) as well as their acceptance of aglycones other than water has provided the abilities of GHs in synthesizing glycosides. Together with desirable physiochemical properties of glycosides and their high commercial values, research interests have been aroused to investigate the synthetic other than the hydrolytic properties of GHs. On the other hand, just like the esterification processes catalyzed by lipases, GH synthetic effectiveness is strongly obstructed by water both thermodynamically and kinetically. Medium engineering by involving organic solvents can be a viable approach to alleviate the obstacles caused by water. However, as native hydrolyases function in water-enriched environments, most GHs display poor catalytic performance in the presence of organic solvents. Some GHs from thermophiles are more tolerant to organic solvents due to their robust folded structures with strong residue interactions. Other than native sources, immobilization, protein engineering, employment of surfactant, and lyophilization have been proved to enhance the GH stability from the native state, which opens up the possibilities for GHs to be employed in unconventional media as synthases.
Key points
• Unconventional media enhance the synthetic ability but destabilize GHs.
• Viable approaches are discussed to improve GH stability from the native state.
• GHs robust in unconventional media can be valuable industrial synthases.



Similar content being viewed by others
References
Arnold FH (1988) Protein design for non-aqueous solvents. Protein Eng Des Sel 2:21–25
Arnold FH (1990) Engineering enzymes for non-aqueous solvents. Trends Biotechnol 8:244–249
Balogh T, Boross L, Kosáry J (2004) Novel reaction systems for the synthesis of O-glucosides by enzymatic reverse hydrolysis. Tetrahedron 60:679–682
Basso A, Ducret A, Gardossi L, Lortie R (2002) Synthesis of octyl glucopyranoside by almond β-glucosidase adsorbed onto Celite R-640®. Tetrahedron Lett 43:2005–2008
Batra J, Mishra S (2013) Organic solvent tolerance and thermostability of a β-glucosidase co-engineered by random mutagenesis. J Mol Catal B Enzym 96:61–66
Bayón C, Cortés Á, Berenguer J, Hernáiz MJ (2013) Highly efficient enzymatic synthesis of Galβ-(1→3)-GalNAc and Galβ-(1→3)-GlcNAc in ionic liquids. Tetrahedron 69:4973–4978
Biswas R, Pal SK (2004) Caging enzyme function: α-chymotrypsin in reverse micelle. Chem Phys Lett 387:221–226
Bohlin C, Praestgaard E, Baumann MJ, Borch K, Praestgaard J, Monrad RN, Westh P (2013) A comparative study of hydrolysis and transglycosylation activities of fungal β-glucosidases. Appl Microbiol Biotechnol 97:159–169
Chen H, Yang S, Xu A, Jiang R, Tang Z, Wu J, Zhu L, Liu S, Chen X, Lu Y (2019) Insight into the glycosylation and hydrolysis kinetics of alpha-glucosidase in the synthesis of glycosides. Appl Microbiol Biotechnol 103:9423–9432
Chothia C, Lesk AM (1986) The relation between the divergence of sequence and structure in proteins. EMBO J 5:823–826
Chu J, Wu X, Li B, He B (2014) Efficient glucosylation of flavonoids by organic solvent-tolerant Staphylococcus saprophyticus CQ16 in aqueous hydrophilic media. J Mol Catal B Enzym 99:8–13
Cruz-Guerrero AE, Gómez-Ruiz L, Viniegra-González G, Bárzana E, García-Garibay M (2006) Influence of water activity in the synthesis of galactooligosaccharides produced by a hyperthermophilic β-glycosidase in an organic medium. Biotechnol Bioeng 93:1123–1129
Davies GJ, Gloster TM, Henrissat B (2005) Recent structural insights into the expanding world of carbohydrate-active enzymes. Curr Opin Struct Biol 15:637–645
Das R, Mukhopadhyay B (2016) Chemical O-Glycosylations: an overview. ChemistryOpen 5:401–433
De Montalk GP, Remaud-Simeon M, Willemot RM, Sarçabal P, Planchot V, Monsan P (2000) Amylosucrase from Neisseria polysaccharea: novel catalytic properties. FEBS Lett 471:219–223
De Winter K, Šimčíková D, Schalck B, Weignerová L, Pelantova H, Soetaert W, Desmet T, Křen V (2013a) Chemoenzymatic synthesis of α-L-rhamnosides using recombinant α-L-rhamnosidase from Aspergillus terreus. Bioresour Technol 147:640–644
De Winter K, Verlinden K, Křen V, Weignerová L, Soetaert W, Desmet T (2013b) Ionic liquids as cosolvents for glycosylation by sucrose phosphorylase: balancing acceptor solubility and enzyme stability. Green Chem 15:1949–1955
Dong G, Vieille C, Savchenko A, Zeikus JG (1997) Cloning, sequencing, and expression of the gene encoding extracellular alpha-amylase from Pyrococcus furiosus and biochemical characterization of the recombinant enzyme. Appl Environ Microbiol 63:3569–3576
Doukyu N, Ogino H (2010) Organic solvent-tolerant enzymes. Biochem Eng J 48:270–282
Dror A, Shemesh E, Dayan N, Fishman A (2014) Protein engineering by random mutagenesis and structure-guided consensus of Geobacillus stearothermophilus lipase T6 for enhanced stability in methanol. Appl Environ Microbiol 80:1515–1527
Ducret A, Carrière JF, Trani M, Lortie R (2002a) Enzymatic synthesis of octyl glucoside catalyzed by almond β-glucosidase in organic media. Can J Chem 80:653–656
Ducret A, Trani M, Lortie R (2002b) Screening of various glycosidases for the synthesis of octyl glucoside. Biotechnol Bioeng 77:752–757
Ducret A, Trani M, Lortie R (2006) Comparison between various commercial sources of almond β-glucosidase for the production of alkyl glucosides. J Mol Catal B Enzym 38:91–94
Earle MJ, Esperança JM, Gilea MA, Lopes JNC, Rebelo LP, Magee JW, Seddon KR, Widegren JA (2006) The distillation and volatility of ionic liquids. Nature 439:831–834
Fang W, Yang Y, Zhang X, Yin Q, Zhang X, Wang X, Fang Z, Yazhong X (2016) Improve ethanol tolerance of β-glucosidase Bgl1A by semi-rational engineering for the hydrolysis of soybean isoflavone glycosides. J Biotechnol 227:64–71
Ferdjani S, Ionita M, Roy B, Dion M, Djeghaba Z, Rabiller C, Tellier C (2011) Correlation between thermostability and stability of glycosidases in ionic liquid. Biotechnol Lett 33:1215–1219
Fitzpatrick PA, Klibanov AM (1991) How can the solvent affect enzyme enantioselectivity? J Am Chem Soc 113:3166–3171
Forsyth SA, MacFarlane DR, Thomson RJ, Von Itzstein M (2002) Rapid, clean, and mild O-acetylation of alcohols and carbohydrates in an ionic liquid. Chem Commun 33:714–715
Fukushima T, Mizuki T, Echigo A, Inoue A, Usami R (2005) Organic solvent tolerance of halophilic α-amylase from a Haloarchaeon, Haloarcula sp. strain S-1. Extremophiles 9:85–89
García-Garibay M, López-Munguía A, Barzana E (2000) Alcoholysis and reverse hydrolysis reactions in organic one-phase system with a hyperthermophilic β-glycosidase. Biotechnol Bioeng 69:627–632
Gargouri M, Smaali I, Maugard T, Legoy MD, Marzouki N (2004) Fungus β-glycosidases: immobilization and use in alkyl-β-glycoside synthesis. J Mol Catal B Enzym 29:89–94
Goedl C, Sawangwan T, Wildberger P, Nidetzky B (2010) Sucrose phosphorylase: a powerful transglucosylation catalyst for synthesis of α-D-glucosides as industrial fine chemicals. Biocatal Biotransform 28:10–21
Graebin NG, Schöffer JDN, Andrades DD, Hertz PF, Ayub MA, Rodrigues RC (2016) Immobilization of glycoside hydrolase families GH1, GH13, and GH70: state of the art and perspectives. Molecules 21:1074
Griebenow K, Klibanov AM (1996) On protein denaturation in aqueous-organic mixtures but not in pure organic solvents. J Am Chem Soc 118:11695–11700
Guo D, Xu Y, Kang Y, Han S, Zheng S (2016) Synthesis of octyl-β-D-glucopyranoside catalyzed by Thai rosewood β-glucosidase-displaying Pichia pastoris in an aqueous/organic two-phase system. Enzym Microb Technol 85:90–97
Gupta A, Ray S, Kapoor S, Khare SK (2008) Solvent-stable Pseudomonas aeruginosa PseA protease gene: identification, molecular characterization, phylogenetic and bioinformatic analysis to study reasons for solvent stability. J Mol Microbiol Biotechnol 15:234–243
Han R, Liu L, Shin HD, Chen RR, Du G, Chen J (2013) Site-saturation engineering of lysine 47 in cyclodextrin glycosyltransferase from Paenibacillus macerans to enhance substrate specificity towards maltodextrin for enzymatic synthesis of 2-O-D-glucopyranosyl-L-ascorbic acid (AA-2G). Appl Microbiol Biotechnol 97:5851–5860
Hansson T, Adlercreutz P (2001) Enhanced transglucosylation/hydrolysis ratio of mutants of Pyrococcus furiosus β-glucosidase: effects of donor concentration, water content, and temperature on activity and selectivity in hexanol. Biotechnol Bioeng 75:656–665
Hansson T, Adlercreutz P (2002) Enzymatic synthesis of hexyl glycosides from lactose at low water activity and high temperature using hyperthermostable β-glycosidases. Biocatal Biotransform 20:167–178
Hao J, Berry A (2004) A thermostable variant of fructose bisphosphate aldolase constructed by directed evolution also shows increased stability in organic solvents. Protein Eng Des Sel 17:689–697
Hari Krishna S, Karanth NG (2002) Lipases and lipase-catalyzed esterification reactions in nonaqueous media. Catal Rev 44:499–591
Hayes MR, Pietruszka J (2017) Synthesis of glycosides by glycosynthases. Molecules 22:1434
Henrissat B (1991) A classification of glycosyl hydrolases based on amino acid sequence similarities. Biochem J 280:309–316
Hoffmann C, Grey C, Pinelo M, Woodley JM, Daugaard AE, Adlercreutz P (2020) Improved alkyl glycoside synthesis by trans-glycosylation through tailored microenvironments of immobilized β-Glucosidase. ChemPlusChem 85:137–141
Hong J, Tamaki H, Kumagai H (2006) Unusual hydrophobic linker region of β-glucosidase (BGLII) from Thermoascus aurantiacus is required for hyper-activation by organic solvents. Appl Microbiol Biotechnol 73:80–88
Hronská H, Mastihuba V, Tokošová S, Rosenberg M (2016) Semicontinual synthesis of alkyl galactosides using β-galactosidase entrapped in polyvinylalcohol hydrogel. Biocatal. Biotransform. 34:219–225
Irazoqui G, Giacomini C, Batista-Viera F, Brena BM, Cardelle-Cobas A, Corzo N, Jimeno ML (2009) Characterization of galactosyl derivatives obtained by transgalactosylation of lactose and different polyols using immobilized β-galactosidase from Aspergillus oryzae. J Agric Food Chem 57:11302–11307
Ismail A, Ghoul M (1996) Enzymatic synthesis of butyiglycosides by glycosidases. Biotechnol Lett 18:1199–1204
Iyer PV, Ananthanarayan L (2008) Enzyme stability and stabilization—aqueous and non-aqueous environment. Process Biochem 43:1019–1032
Jeong JW, Seo DH, Jung JH, Park JH, Baek NI, Kim MJ, Park CS (2014) Biosynthesis of glucosyl glycerol, a compatible solute, using intermolecular transglycosylation activity of amylosucrase from Methylobacillus flagellatus KT. Appl Biochem Biotechnol 173:904–917
Jiang JR, Yuan S, Ding JF, Zhu SC, Xu HD, Chen T, Cong XD, Xu WP, Ye H, Dai,YJ (2008) Conversion of puerarin into its 7-O-glycoside derivatives by Microbacterium oxydans (CGMCC 1788) to improve its water solubility and pharmacokinetic properties. Appl Microbiol Biotechnol 81: 647–657
Kaftzik N, Wasserscheid P, Kragl U (2002) Use of ionic liquids to increase the yield and enzyme stability in the β-galactosidase catalysed synthesis of N-acetyllactosamine. Org Process Res Dev 6:553–557
Kaper T, Brouns SJ, Geerling AC, Vos WMD, Van der Oost J (2002) DNA family shuffling of hyperthermostable β-glycosidases. Biochem J 368:461–470
Karav S, Cohen JL, Barile D, de Moura BJMLN (2017) Recent advances in immobilization strategies for glycosidases. Biotechnol Prog 33:104–112
Kieboom AP (1988) Enzymes that do not work in organic solvents: too polar substrates give too tight enzyme-product complexes. Recl Trav Chim Pays-Bas 107:347–348
Kleywegt GJ, Zou JY, Divne C, Davies GJ, Sinning I, Ståhlberg J, Reinikainen T, Srisodsuk M, Teeri TT, Jones TA (1997) The crystal structure of the catalytic core domain of endoglucanase I from Trichoderma reesei at 3.6 Å resolution, and a comparison with related enzymes. J Mol Biol 272:383–397
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246
Kobayashi T, Adachi S, Nakanishi K, Matsuno R (2000) Synthesis of alkyl glycosides through β-glucosidase-catalyzed condensation in an aqueous–organic biphasic system and estimation of the equilibrium constants for their formation. J Mol Catal B Enzym 11:13–21
Koshland DE Jr (1953) Stereochemistry and the mechanism of enzymatic reactions. Biol Rev 28:416–436
Kosmulski M, Gustafsson J, Rosenholm JB (2004) Thermal stability of low temperature ionic liquids revisited. Thermochim Acta 412:47–53
Kouptsova OS, Klyachko NL, Levashov AV (2001) Synthesis of alkyl glycosides catalyzed by β-glycosidases in a system of reverse micelles. Russ J Bioorg Chem 27:380–384
Kragl U, Eckstein M, Kaftzik N (2002) Enzyme catalysis in ionic liquids. Curr Opin Biotechnol 13:565–571
Kurosu J, Sato T, Yoshida K, Tsugane T, Shimura S, Kirimura K, Kino K, Usami S (2002) Enzymatic synthesis of α-arbutin by α-anomer-selective glucosylation of hydroquinone using lyophilized cells of Xanthomonas campestris WU-9701. J Biosci Bioeng 93:328–330
Kwon T, Kim CT, Lee JH (2007) Transglucosylation of ascorbic acid to ascorbic acid 2-glycoside by a recombinant sucrose phosphorylase from Bifidobacterium longum. Biotechnol Lett 29:611–615
Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures, functions, and mechanisms. Annu Rev Biochem 77:521–555
Laroute V, Willemot RM (1992a) Glucoside synthesis by glucoamylase or\-glucosidase in organic solvents. Biotechnol Lett 14:169–174
Laroute V, Willemot RM (1992b) Effect of organic solvents on stability of two glycosidases and on glucoamylase-catalysed oligosaccharide synthesis. Enzym Microb Technol 14:528–534
Lee TM, Farrow MF, Arnold FH, Mayo SL (2011) A structural study of Hypocrea jecorina Cel5A. Protein Sci 20:1935–1940
Li Z, Han H, Wang B, Gao J, Zhu B, Peng R, Yao Q (2017) Transglucosylation of ascorbic acid to ascorbic acid 2-glycoside by a truncated version of α-glucosidase from Aspergillus niger. J Food Biochem 41:e12432
Liu Q, Janssen MH, van Rantwijk F, Sheldon RA (2005) Room-temperature ionic liquids that dissolve carbohydrates in high concentrations. Green Chem 7:39–42
Liu QP, Sulzenbacher G, Yuan H, Bennett EP, Pietz G, Saunders K, Spence J, Nudelman E, Levery SB, White T, Neveu JM, Lane WS, Bourne Y, Olsson ML, Henrissat B, Clausen H (2007) Bacterial glycosidases for the production of universal red blood cells. Nat Biotechnol 25:454–464
Lundemo P, Adlercreutz P, Karlsson EN (2013) Improved transferase/hydrolase ratio through rational design of a family 1 β-glucosidase from Thermotoga neapolitana. Appl Environ Microbiol 79:3400–3405
Martearena MR, Blanco S, Ellenrieder G (2003) Synthesis of alkyl-α-L-rhamnosides by water soluble alcohols enzymatic glycosylation. Bioresour Technol 90:297–303
Mateo C, Palomo JM, Fernandez-Lorente G, Guisan JM, Fernandez-Lafuente R (2007) Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym Microb Technol 40:1451–1463
Mladenoska I, Grey CE, Winkelhausen E, Kuzmanova S, Adlercreutz P (2007) Competition between transglycosylation and hydrolysis in almond β-glucosidase-catalyzed conversion of p-nitrophenyl-β-d-glucoside in monophasic water/alcohol mixtures. Biocatal Biotransform 25:382–385
Moracci M, Trincone A, Cobucci-Ponzano B, Perugino G, Ciaramella M, Rossi M (2001) Enzymatic synthesis of oligosaccharides by two glycosyl hydrolases of Sulfolobus solfataricus. Extremophiles 5:145–152
Moradi SV, Hussein WM, Varamini P, Simerska P, Toth I (2016) Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem Sci 7:2492–2500
Mori T, Okahata Y (1997) A variety of lipid-coated glycoside hydrolases as effective glycosyl transfer catalysts in homogeneous organic solvents. Tetrahedron Lett 38:1971–1974
Munoz FJ, André S, Gabius HJ, Sinisterra JV, Hernáiz MJ, Linhardt RJ (2009) Green glycosylation using ionic liquid to prepare alkyl glycosides for studying carbohydrate–protein interactions by SPR. Green Chem 11:373–379
Nagatomo H, Matsushita YI, Sugamoto K, Matsui T (2005) Preparation and properties of gelatin-immobilized β-glucosidase from Pyrococcus furiosus. Biosci Biotechnol Biochem 69:128–136
Nakagawa H, Dobashi Y, Sato T, Yoshida K, Tsugane T, Shimura S, Kirimura K, Kino K, Usami S (2000) α-Anomer-selective glucosylation of menthol with high yield through a crystal accumulation reaction using lyophilized cells of Xanthomonas campestris WU-9701. J Biosci Bioeng 89:138–144
Naundorf A, Caussette M, Ajisaka K (1998) Characterization of the immobilized β-galactosidase C from Bacillus circulans and the production of β (1→ 3)-linked disaccharides. Biosci Biotechnol Biochem 62:1313–1317
Ogino H, Nakagawa S, Shinya K, Muto T, Fujimura N, Yasuda M, Ishikawa H (2000) Purification and characterization of organic solvent-stable lipase from organic solvent-tolerant Pseudomonas aeruginosa LST-03. J Biosci Bioeng 89:451–457
Ogino H, Uchiho T, Doukyu N, Yasuda M, Ishimi K, Ishikawa H (2007) Effect of exchange of amino acid residues of the surface region of the PST-01 protease on its organic solvent-stability. Biochem Biophys Res Commun 358:1028–1033
Ojima T, Saburi W, Yamamoto T, Kudo T (2012) Characterization of Halomonas sp. strain H11 α-glucosidase activated by monovalent cations and its application for efficient synthesis of α-D-Glucosylglycerol. Appl Environ Microbiol 78:1836–1845
Papanikolaou S (2001) Enzyme-catalyzed synthesis of alkyl-β-glucosides in a water–alcohol two-phase system. Bioresour Technol 77:157–161
Park S, Kazlauskas RJ (2003) Biocatalysis in ionic liquids–advantages beyond green technology. Curr Opin Biotechnol 14:432–437
Pavlidis IV, Gournis D, Papadopoulos GK, Stamatis H (2009) Lipases in water-in-ionic liquid microemulsions: structural and activity studies. J Mol Catal B Enzym 60:50–56
Plaza M, Pozzo T, Liu J, Gulshan Ara KZ, Turner C, Nordberg Karlsson E (2014) Substituent effects on in vitro antioxidizing properties, stability, and solubility in flavonoids. J Agric Food Chem 62:3321–3333
Rather MY, Mishra S, Chand S (2010) β-Glucosidase catalyzed synthesis of octyl-β-D-glucopyranoside using whole cells of Pichia etchellsii in micro aqueous media. J Biotechnol 150:490–496
Ravet C, Thomas D, Legoy MD (1993) Gluco-oligosaccharide synthesis by free and immobilized β-glucosidase. Biotechnol Bioeng 42:303–308
Rye CS, Withers SG (2000) Glycosidase mechanisms. Curr Opin Chem Biol 4:573–580
Saeki H, Inoue K (1997) Improved solubility of carp myofibrillar proteins in low ionic strength medium by glycosylation. J Agric Food Chem 45:3419–3422
Saeki H, Tanabe M (1999) Change in solubility of carp myofibrillar protein by glycosylation with ribose. Fish Sci 65:967–968
Sato D, Eshita Y, Katsuragi H, Hamada H, Shimoda K, Kubota N (2012) Glycosylation of vanillin and 8-nordihydrocapsaicin by cultured Eucalyptus perriniana cells. Molecules 17:5013–5020
Schrader A, Siefken W, Kueper T, Breitenbach U, Gatermann C, Sperling G, Biernoth T, Scherner C, Stäb F, Wenck H, Wittern KP (2012) Effects of glyceryl glycoside on AQP3 expression, barrier function and hydration of human skin. Skin Pharmacol Physiol 25:192–199
Sellek GA, Chaudhuri JB (1999) Biocatalysis in organic media using enzymes from extremophiles. Enzym Microb Technol 25:471–482
Sinclair AM, Elliott S (2005) Glycoengineering: the effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci 94:1626–1635
Solá RJ, Griebenow K (2010) Glycosylation of therapeutic proteins. BioDrugs 24:9–21
Song JK, Rhee JS (2001) Enhancement of stability and activity of phospholipase A1 in organic solvents by directed evolution. Biochim Biophys Acta Protein Struct Mol Enzymol 1547:370–378
Sun H, Xue Y, Lin Y (2014) Enhanced catalytic efficiency in quercetin-4′-glucoside hydrolysis of Thermotoga maritima β-glucosidase A by site-directed mutagenesis. J Agric Food Chem 62:6763–6770
Svensson I, Wehtje E, Adlercreutz P, Mattiasson B (1994) Effects of water activity on reaction rates and equilibrium positions in enzymatic esterifications. Biotechnol Bioeng 44:549–556
Takahashi H, Li B, Sasaki T, Miyazaki C, Kajino T, Inagaki S (2001) Immobilized enzymes in ordered mesoporous silica materials and improvement of their stability and catalytic activity in an organic solvent. Microporous Mesoporous Mater 44:755–762
Thilakarathna SH, Rupasinghe HP (2013) Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 5:3367–3387
Thumar JT, Singh SP (2009) Organic solvent tolerance of an alkaline protease from salt-tolerant alkaliphilic Streptomyces clavuligerus strain Mit-1. J Ind Microbiol Biotechnol 36:211–218
van der Veen BA, van Alebeek GJW, Uitdehaag JC, Dijkstra BW, Dijkhuizen L (2000) The three transglycosylation reactions catalyzed by cyclodextrin glycosyltransferase from Bacillus circulans (strain 251) proceed via different kinetic mechanisms. Eur J Biochem 267:658–665
van Rantwijk F, Sheldon RA (2007) Biocatalysis in ionic liquids. Chem Rev 107:2757–2785
Van Rantwijk F, Woudenberg-van Oosterom M, Sheldon RA (1999) Glycosidase-catalysed synthesis of alkyl glycosides. J Mol Catal B Enzym 6:511–532
Vera C, Guerrero C, Wilson L, Illanes A (2017) Synthesis of propyl-β-D-galactoside with free and immobilized β-galactosidase from Aspergillus oryzae. Process Biochem 53:162–171
Vic G, Biton J, Le Beller D, Michel JM, Thomas D (1995) Enzymatic glucosylation of hydrophobic alcohols in organic medium by the reverse hydrolysis reaction using almond-β-D-glucosidase. Biotechnol Bioeng 46:109–116
Vic G, Crout DH (1994) Synthesis of glucosidic derivatives with a spacer arm by reverse hydrolysis using almond β-D-glucosidase. Tetrahedron Asymmetry 5:2513–2516
Vic G, Hastings JJ, Crout DH (1996) Glycosidase-catalysed synthesis of glycosides by an improved procedure for reverse hydrolysis: application to the chemoenzymatic synthesis of galactopyranosyl-(1→4)-O-α-galactopyranoside derivatives. Tetrahedron Asymmetry 7:1973–1984
Vic G, Thomas D, Crout DH (1997) Solvent effect on enzyme-catalyzed synthesis of β-D-glucosides using the reverse hydrolysis method: application to the preparative-scale synthesis of 2-hydroxybenzyl and octyl β-D-glucopyranosides. Enzym Microb Technol 20:597–603
Vulfson EN, Patel R, Law BA (1990) Alkyl-β-glucoside synthesis in a water-organic two-phase system. Biotechnol Lett 12:397–402
Wang F, Ma Y, Liu YH, Zhang X, Zhang F, Linhardt RJ (2017) Improved octyl glucoside synthesis using immobilized β-glucosidase on PA-M with reduced glucose surplus inhibition. Biocatal Biotransform 35:349–362
Wang Q, Yu H, Zhao N, Li C, Shang Y, Liu H, Xu J (2012) Significantly improved equilibrium yield of long-chain alkyl glucosides via reverse hydrolysis in a water-poor system using cross-linked almond meal as a cheap and robust biocatalyst. Chin J Catal 33:275–280
Wei W, Qi D, Zhao HZ, Lu ZX, Lv F, Bie X (2013) Synthesis and characterisation of galactosyl glycerol by β-galactosidase catalysed reverse hydrolysis of galactose and glycerol. Food Chem 141:3085–3092
Wu X, Chu J, Wu B, Zhang S, He B (2013) An efficient novel glycosylation of flavonoid by β-fructosidase resistant to hydrophilic organic solvents. Bioresour Technol 129:659–662
Xu L, Liu X, Yin Z, Liu Q, Lu L, Xiao M (2016) Site-directed mutagenesis of α-l-rhamnosidase from Alternaria sp. L1 to enhance synthesis yield of reverse hydrolysis based on rational design. Appl Microbiol Biotechnol 100:10385–10394
Yamamoto I, Muto N, Nagata E, Nakamura T, Suzuki Y (1990) Formation of a stable L-ascorbic acid α-glucoside by mammalian α-glucosidase-catalyzed transglucosylation. Biochim Biophys Acta, Gen Subj 1035:44–50
Yang Y, Zhang X, Yu B (2015) O-Glycosylation methods in the total synthesis of complex natural glycosides. Nat Prod Rep 32:1331–1355
Zhu L, Jiang D, Zhou Y, Lu Y, Fan Y, Chen X (2019a) Batch-feeding whole-cell catalytic synthesis of α-arbutin by amylosucrase from Xanthomonas campestris. J Ind Microbiol Biotechnol 46:759–767
Zhu L, Xu M, Lu C, Chen L, Xu A, Fang J, Chen H, Lu Y, Fan Y, Chen X (2019b) Optimization of whole-cell biotransformation for scale-up production of α-arbutin from hydroquinone by the use of recombinant Escherichia coli. AMB Express 9:94
Zumárraga M, Bulter T, Shleev S, Polaina J, Martínez-Arias A, Plou FJ, Ballesteros A, Alcalde M (2007) In vitro evolution of a fungal laccase in high concentrations of organic cosolvents. Chem Biol 14:1052–1064
Acknowledgments
We are grateful to the National Natural Science Foundation of China and Key Research & Development in Zhejiang in encouraging us for the studies in the glycosylation area.
Funding
This study was funded by the National Natural Science Foundation of China (No. 21908196) and Key Research & Development in Zhejiang (No. 2019C01085).
Author information
Authors and Affiliations
Contributions
All authors have their own contributions to this work. The first author, Dr. Hanchi Chen, was in charge of the study including writing the manuscript. Ms. Xiao Jin has been helped in collecting and organizing materials. Dr. Linjiang Zhu, Dr. Yuele Lu, Dr. Zhi Ma, and Dr. Shijie Liu have been providing valuable advices. Dr. Xiaolong Chen has founded the whole study as well as providing research advices.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chen, H., Jin, X., Zhu, L. et al. Glycosyl hydrolase catalyzed glycosylation in unconventional media. Appl Microbiol Biotechnol 104, 9523–9534 (2020). https://doi.org/10.1007/s00253-020-10924-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10924-1