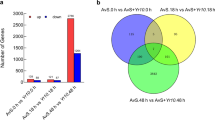
Abstract
The pigeonpea wild relative Cajanus platycarpus is resistant to Helicoverpa armigera, one of the major pests responsible for yield losses in Cajanus cajan. Deciphering the molecular mechanism underlying host plant resistance is pertinent to identify proteins that aid in the mitigation of the insect pest. The present study adopted comparative proteomics as a tool to interpret the resistance mechanism(s) in C. platycarpus vis-à-vis C. cajan during continued herbivory (up to 96 h). Over-representation analysis of the differentially expressed proteins implicated a multi-dimensional resistance response accomplished by both physical and chemical barriers in C. platycarpus. While the chemical basis for resistance was depicted by the upregulation of proteins playing a rate limiting role in the phenylpropanoid pathway, the physical basis was provided by the regulation of proteins involved in microtubule assembly and synthesis of lignins. Upregulation of proteins in the polyamine pathway indicated the role of metabolite conjugates to be negatively affecting herbivore growth. Reallocation of resources and diversion of metabolic flux to support the production of secondary metabolites could be the probable approach in the wild relative against herbivory. Our study provided deeper insights into the pod borer resistance mechanism in C. platycarpus for utility in crop improvement.
Key points
• Pod borer resistance in Cajanus platycarpus is multi-dimensional.
• Pod borer resistance has been arbitrated to cell wall rigidity and secondary metabolites.
• Phenylpropanoid pathway derivatives apparently shaped the plant chemical defense against pod borer.








Similar content being viewed by others
References
Bader GD, Hogue CW (2003) An automated method for finding molecular complexes in large protein interaction networks. BMC Bioinforma 4:2
Brozynska M, Furtado A, Henry RJ (2016) Genomics of crop wild relatives: expanding the gene pool for crop improvement. Plant Biotechnol J 14:070–1085
Chang J, Xu Z, Li M, Yang M, Qin H, Yang J, Wu S (2019) Spatiotemporal cytoskeleton organizations determine morphogenesis of multicellular trichomes in tomato. PLoS Genet 15:e1008438
Chen J, Ullah H, Tu X, Zhang Z (2019) Understanding the genetic mechanism of resistance in aphid-treated alfalfa (Medicago sativa L.) through proteomic analysis. 3 Biotech 9:241
Cox J, Mann M (2008) MaxQuant enables high peptide identification rates, individualized ppb-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26:1367–1372
Coleman HD, Park JY, Nair R, Chapple C, Mansfield SD (2008) RNAi-mediated suppression of p-coumaroyl-CoA 3′-hydroxylase in hybrid poplar impacts lignin deposition and soluble secondary metabolism. Proc Natl Acad Sci U S A 105:4501–4506
Dempewolf H, Baute G, Anderson J, Kilian B, Smith C, Guarino L (2017) Past and future use of wild relatives in crop breeding. Crop Sci 57:1070–1082
Gaquerel E, Gulati J, Baldwin IT (2014) Revealing insect herbivory-induced phenolamide metabolism: from single genes to metabolic network plasticity analysis. Plant J 79:679–692
Glas JJ, Schimmel BC, Alba JM, Escobar-Bravo R, Schuurink RC, Kant MR (2012) Plant glandular trichomes as targets for breeding or engineering of resistance to herbivores. Int J Mol Sci 13:17077–17103
Green PWC, Sharma HC, Stevenson PC, Simmonds (2006) MSJ Susceptibility of pigeonpea and some of its wild relatives to predation by Helicoverpa armigera: implications for breeding resistant cultivars. Aust J Agric Res 57:831–836
Gu Y, Zavaliev R, Dong X (2017) Membrane trafficking in plant immunity. Mol Plant 10:1026–1034
Guillet G, De Luca V (2005) Wound-inducible biosynthesis of phytoalexin hydroxycinnamic acid amides of tyramine in tryptophan and tyrosine decarboxylase transgenic tobacco lines. Plant Physiol 137:692–699
Hanley ME, Lamont BB, Fairbanks MM, Rafferty CM (2007) Plant structural traits and their role in anti-herbivore defence. Perspect Plant Ecol Evol Syst 8:157–178
Kang X, Wang L, Guo Y, ul Arifeen MZ, Cai X, Xue Y, Bu Y, Wang G, Liu CA (2019) Comparative transcriptomic and proteomic analysis of hexaploid wheat’s responses to colonization by Bacillus velezensis and Gaeumannomyces graminis, both separately and combined. Mol Plant-Microbe Interact 32:1336–1347
Kaur H, Heinzel N, Schöttner M, Baldwin IT, Gális I (2010) R2R3-NaMYB8 regulates the accumulation of phenylpropanoid-polyamine conjugates, which are essential for local and systemic defense against insect herbivores in Nicotiana attenuata. Plant Physiol 152:1731–1747
Kohl M, Wiese S, Warscheid B (2011) Cytoscape: software for visualization and analysis of biological networks. In: Hamacher M, Eisenacher M, Stephan C (eds) Data mining in proteomics, 1st edn. Humana Press, Totowa, pp 291–303
Kumar NR, Rathinam M, Singh S, Kesiraju K, Muniyandi V, Singh NK, Dash PK, Sreevathsa R (2019) Assessment of pigeonpea (Cajanus cajan L.) transgenics expressing Bt ICPs, Cry2Aa and Cry1AcF under nethouse containment implicated an effective control against herbivory by Helicoverpa armigera (Hübner). Pest Manag Sci. https://doi.org/10.1002/ps.5722
Li J, Zhu L, Hull JJ, Liang S, Daniell H, Jin S, Zhang X (2016a) Transcriptome analysis reveals a comprehensive insect resistance response mechanism in cotton to infestation by the phloem feeding insect Bemisia tabaci (whitefly). Plant Biotechnol J 14:1956–1975
Li T, Cofer T, Engelberth M, Engelberth J (2016b) Defense priming and jasmonates: a role for free fatty acids in insect elicitor-induced long distance signaling. Plants 5:5
Liu Y, Lu S, Liu K, Wang S, Huang L, Guo L (2019) Proteomics: a powerful tool to study plant responses to biotic stress. Plant Methods 15:135
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Lu Y, Chen Q, Bu Y, Luo R, Hao S, Zhang J, Tian J, Yao Y (2017) Flavonoid accumulation plays an important role in the rust resistance of Malus plant leaves. Front Plant Sci 8:1286
Mering CV, Huynen M, Jaeggi D, Schmidt S, Bork P, Snel B (2003) STRING: a database of predicted functional associations between proteins. Nucleic Acids Res 31:258–261
Mishra P, Singh S, Rathinam M, Nandiganti M, Ram Kumar N, Thangaraj A, Thimmegowda V, Krishnan V, Mishra V, Jain N, Rai V, Pattanayak D, Sreevathsa R (2017) Comparative proteomic and nutritional composition analysis of independent transgenic pigeon pea seeds harboring cry1AcF and cry2Aa genes and their non-transgenic counterparts. J Agric Food Chem 65:1395–1400
Moschou PN, Wu J, Cona A, Tavladoraki P, Angelini R, Roubelakis-Angelakis KA (2012) The polyamines and their catabolic products are significant players in the turnover of nitrogenous molecules in plants. J Exp Bot 63:5003–5015
Mula MG, Saxena KB (2010) Lifting the level of awareness on pigeonpea-a global perspective. International Crops Research Institute for the Semi-Arid Tropics, Patancheru
Muthamilarasan M, Singh NK, Prasad M (2019) Multi-omics approaches for strategic improvement of stress tolerance in underutilized crop species: A climate change perspective. Adv Genet 103:1–38
Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu D, Inuganti A, Griss J, Maye G, Eisenacher M, Pérez E (2019) The PRIDE database and related tools and resources in 2019: improving support for quantification data. Nucleic Acids Res 47:442–450
Plett JM, Mathur J, Regan S (2009) Ethylene receptor ETR2 controls trichome branching by regulating microtubule assembly in Arabidopsis thaliana. J Exp Bot 60:3923–3933
Rathinam M, Mishra P, Vasudevan M, Budhwar R, Mahato A, Prabha AL, Singh NK, Rao U, Sreevathsa R (2019a) Comparative transcriptome analysis of pigeonpea, Cajanus cajan (L.) and one of its wild relatives Cajanus platycarpus (Benth.) Maesen. PLoS One 14:e0218731
Rathinam M, Mishra P, Mahato AK, Singh NK, Rao U, Sreevathsa R (2019b) Comparative transcriptome analyses provide novel insights into the differential response of pigeonpea (Cajanus cajan L.) and its wild relative (Cajanus platycarpus (Benth.) Maesen) to herbivory by Helicoverpa armigera (Hübner). Plant Mol Biol 101:163–182
Ritchie ME, Phipson B, Wu DI, Hu Y, Law CW, Shi W, Smyth GK (2015) Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43:47–47
Santiago R, Barros-Rios J, Malvar RA (2013) Impact of cell wall composition on maize resistance to pests and diseases. Int J Mol Sci 14:6960–6980
Scardoni G, Tosadori G, Faizan M, Spoto F, Fabbri F, Laudanna C (2014) Biological network analysis with CentiScaPe: centralities and experimental dataset integration. F1000Res 3:139
Schwachtje J, Baldwin IT (2008) Why does herbivore attack reconfigure primary metabolism? Plant Physiol 146:845–851
Sharma HC, Sujana G, Rao DM (2009) Morphological and chemical components of resistance to pod borer, Helicoverpa armigera in wild relatives of pigeonpea. Arthropod Plant Interact 3:151–161
Singh SP, Singh Y (2001) Control of pod borers on pigeonpea. Indian J Entomol 63:356–359
Sinha P, Saxena RK, Singh VK, Krishnamurthy L, Varshney RK (2015) Selection and validation of housekeeping genes as reference for gene expression studies in pigeonpea (Cajanus cajan) under heat and salt stress conditions. Front Plant Sci 6:1071
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A 102:15545–15550
Sujana G, Sharma HC, Manohar Rao D (2008) Antixenosis and antibiosis components of resistance to pod borer Helicoverpa armigera in wild relatives of pigeonpea. Int J Trop Insect Sci 28:191–200
Tauzin AS, Giardina T (2014) Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front Plant Sci 5:293
Taylor TA (1978) Maruca testulalis: an important pest of tropical grain legumes. In: Singh SR, Van Emden HF, Taylor TA (eds) Pests of Grain Legumes: Ecology and Control, 1st edn. Academic Press, London, pp 193–200
Türker C, Akal F, Schlapbach R (2011) Life sciences data and application integration with B-fabric. J Integr Bioinform 8:49–58
Tyanova S, Temu T, Cox J (2016) The MaxQuant computational platform for mass spectrometry-based shotgun proteomics. Nat Protoc 11:2301–2319
Wang X, Lu J, Chen H, Shan Z, Shen X, Duan B, Zhang C, Yang Z, Zhang X, Qiu D, Chen S (2017) Comparative analyses of transcriptome and proteome in response to cotton bollworm between a resistant wild soybean and a susceptible soybean cultivar. Plant Cell Tissue Organ Cult 129:511–520
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer, New York
Wiśniewski JR, Zougman A, Nagaraj N, Mann M (2009) Universal sample preparation method for proteome analysis. Nat Methods 6:359–362
Wolski W, Grossmann J, Panse C (2018) SRMService - R-Package to Report Quantitative Mass Spectrometry Data. Gitgub. http://github.com/protViz/SRMService. Accessed 26 January 2020
Zhang H, Mittal N, Leamy LJ, Barazani O, Song BH (2017) Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol Appl 10:5–24
Funding
The study was financially supported by DBT-Indo Swiss Collaboration in Biotechnology.
Author information
Authors and Affiliations
Contributions
RoS, BR, UR conceived and designed the experiments; MR, PM conducted the herbivore challenge experiment and extracted the total proteins; BR, LK performed mass spectrometry and data acquisition; JG, WW, CP developed local data base and performed the protein quantification and identification; MR, JG, PM, ST performed downstream analysis and validated the proteome data; MR, BR, JG and RoS wrote the manuscript; RoS, UR, RaS critically reviewed and edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies concerned with experimentation on human or animals.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Rathinam, M., Roschitzki, B., Grossmann, J. et al. Unraveling the proteomic changes involved in the resistance response of Cajanus platycarpus to herbivory by Helicoverpa armigera. Appl Microbiol Biotechnol 104, 7603–7618 (2020). https://doi.org/10.1007/s00253-020-10787-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10787-6