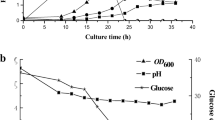
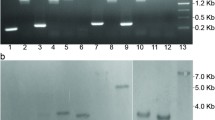
Abstract
Despite the long-term interest in solventogenic clostridia-based ABE (acetone-butanol-ethanol) fermentation, clostridial butanol tolerance and its underlying mechanism remain poorly understood, which is a major obstacle hindering further improvements of this important fermentative process. In this study, a two-component system (TCS), BtrK/BtrR, was identified and demonstrated to positively regulate butanol tolerance and ABE solvent formation in Clostridium acetobutylicum, a representative species of solventogenic clostridia. The transcriptomic analysis results showed that BtrK/BtrR has a pleiotropic regulatory function, affecting a large number of crucial genes and metabolic pathways. Of the differentially expressed genes, btrTM, encoding a putative ABC-type transporter (named BtrTM), was shown to be under the direct control of BtrR, the response regulator of the BtrK/BtrR TCS. Furthermore, BtrTM was shown to contribute to more butanol tolerance (46.5% increase) by overexpression, revealing a novel regulatory mechanism consisting of the BtrK/BtrR TCS and the BtrTM transporter in C. acetobutylicum. Based on these findings, we achieved faster growth and solvent production of C. acetobutylicum by overexpressing BtrK/BtrR or its direct target BtrTM, although no significant improvement in the final butanol titer and yield. These results further confirm the importance of BtrK/BtrR and BtrTM in this organism. Also, of significance, a specific number of btrR-btrT-btrM-btrK-like gene clusters were identified in other Clostridium species, including the pathogens Clostridium perfringens and Clostridium botulinum, indicating a broad role for this regulatory module in the class Clostridia.







Similar content being viewed by others
References
Albanesi D, Mansilla MC, de Mendoza D (2004) The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol 186:2655–2663
Alsaker KV, Spitzer TR, Papoutsakis ET (2004) Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell’s response to butanol stress. J Bacteriol 186:1959–1971
Alsaker KV, Paredes CJ, Papoutsakis ET (2005) Design, optimization and validation of genomic DNA microarrays for examining the Clostridium acetobutylicum transcriptome. Biotechnol Bioprocess Eng 10:432–443
Alsaker KV, Paredes C, Papoutsakis ET (2010) Metabolite stress and tolerance in the production of biofuels and chemicals: gene-expression-based systems analysis of butanol, butyrate, and acetate stresses in the anaerobe Clostridium acetobutylicum. Biotechnol Bioeng 105:1131–1147
Baer SH, Blaschek HP, Smith TL (1987) Effect of butanol challenge and temperature on lipid composition and membrane fluidity of butanol-tolerant Clostridium acetobutylicum. Appl Environ Microbiol 53(12):2854–2861
Bekker M, Teixeira de Mattos MJ, Hellingwerf KJ (2006) The role of two-component regulation systems in the physiology of the bacterial cell. Sci Prog 89(Pt 3–4):213–242
Borden JR, Papoutsakis ET (2007) Dynamics of genomic-library enrichment and identification of solvent tolerance genes for Clostridium acetobutylicum. Appl Environ Microbiol 73:3061–3068
Darkwah K, Nokes SE, Seay JR, Knutson BL (2018) Mechanistic simulation of batch acetone-butanol-ethanol (ABE) fermentation with in situ gas stripping using Aspen Plus (TM). Bioprocess Biosyst Eng 41(9):1283–1294
Darmon E, Noone D, Masson A, Bron S, Kuipers OP, Devine KM, van Dijl JM (2002) A novel class of heat and secretion stress-responsive genes is controlled by the autoregulated CssRS two-component system of Bacillus subtilis. J Bacteriol 184(20):5661–5671
Davidson AL, Dassa E, Orelle C, Chen J (2008) Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol Mol Biol Rev 72:317–364
Dintner S, Staron A, Berchtold E, Petri T, Mascher T, Gebhard S (2011) Coevolution of ABC transporters and two-component regulatory systems as resistance modules against antimicrobial peptides in Firmicutes Bacteria. J Bacteriol 193(15):3851–3862
Fernandez P, Porrini L, Albanesi D, Abriata LA, Dal Peraro M, de Mendoza D, Mansilla MC (2019) Transmembrane prolines mediate signal sensing and decoding in Bacillus subtilis DesK histidine kinase. mBio 10(6):e02564-19
Filippova EV, Tkaczuk KL, Chruszcz M, Xu X, Savchenko A, Edwards A, Minor W (2014) Structural characterization of the putative ABC-type 2 transporter from Thermotoga maritima MSB8. J Struct Funct Genom 15(4):215–222
Fukuchi K, Kasahara Y, Asai K, Kobayashi K, Moriya S, Ogasawara N (2000) The essential two-component regulatory system encoded by yycF and yycG modulates expression of the ftsAZ operon in Bacillus subtilis. Microbiology 146:1573–1583
Hofmann S, Januliene D, Mehdipour AR, Thomas C, Stefan E, Bruchert S, Kuhn BT, Geertsma ER, Hummer G, Tampe R, Moeller A (2019) Conformation space of a heterodimeric ABC exporter under turnover conditions. Nature 571:580–583
Hunger K, Beckering CL, Marahiel MA (2004) Genetic evidence for the temperature-sensing ability of the membrane domain of the Bacillus subtilis histidine kinase DesK. FEMS Microbiol Lett 230:41–46
Janssen H, Grimmler C, Ehrenreich A, Bahl H, Fischer RJ (2012) A transcriptional study of acidogenic chemostat cells of Clostridium acetobutylicum—solvent stress caused by a transient n-butanol pulse. J Biotechnol 161:354–365
Jones AJ, Venkataramanan KP, Papoutsakis T (2016) Overexpression of two stress-responsive, small, non-coding RNAs, 6S and tmRNA, imparts butanol tolerance in Clostridium acetobutylicum. FEMS Microbiol Lett 363(8):fnw063
Lee SY, Park JH, Jang SH, Nielsen LK, Kim J, Jung KS (2008) Fermentative butanol production by Clostridia. Biotechnol Bioeng 101(2):209–228
Li SB, Qian Y, Liang ZW, Guo Y, Zhao MM, Pang ZW (2016) Enhanced butanol production from cassava with Clostridium acetobutylicum by genome shuffling. World J Microbiol Biotechnol 32:53
Liao ZP, Zhang YN, Luo S, Suo YK, Zhang SZ, Wang JF (2017) Improving cellular robustness and butanol titers of Clostridium acetobutylicum ATCC 824 by introducing heat shock proteins from an extremophilic bacterium. J Biotechnol 252:1–10
Liu XB, Gu QY, Yu XB (2013) Repetitive domestication to enhance butanol tolerance and production in Clostridium acetobutylicum through artificial simulation of bio-evolution. Bioresour Technol 130:638–643
Liu J, Lin QL, Chai XY, Luo YC, Guo T (2018) Enhanced phenolic compounds tolerance response of Clostridium beijerinckii NCIMB 8052 by inactivation of Cbei_3304. Microb Cell Factories 17(1):35
Locher KP (2016) Mechanistic diversity in ATP-binding cassette (ABC) transporters. Nat Struct Mol Biol 23:487–493
Mann MS, Dragovic Z, Schirrmacher G, Lutke-Eversloh T (2012) Over-expression of stress protein-encoding genes helps Clostridium acetobutylicum to rapidly adapt to butanol stress. Biotechnol Lett 34(9):1643–1649
Martin JF (2004) Phosphate control of the biosynthesis of antibiotics and other secondary metabolites is mediated by the PhoR-PhoP system: an unfinished story. J Bacteriol 186(16):5197–5201
Martinez JL, Sanchez MB, Martinez-Solano L, Hernandez A, Garmendia L, Fajardo A, Alvarez-Ortega C (2009) Functional role of bacterial multidrug efflux pumps in microbial natural ecosystems. FEMS Microbiol Rev 33:430–449
Meehl M, Herbert S, Gotz F, Cheung A (2007) Interaction of the GraRS two-component system with the VraFG ABC transporter to support vancomycin-intermediate resistance in Staphylococcus aureus. Antimicrob Agents Chemother 51(8):2679–2689
Ngernsombat C, Sreesai S, Harnvoravongchai P, Chankhamhaengdecha S, Janvilisri T (2017) CD2068 potentially mediates multidrug efflux in Clostridium difficile. Sci Rep 7(1):9982
Patakova P, Kolek J, Sedlar K, Koscova P, Branska B, Kupkova K, Paulova L, Provaznik I (2018) Comparative analysis of high butanol tolerance and production in clostridia. Biotechnol Adv 36(3):721–738
Rees DC, Johnson E, Lewinson O (2009) ABC transporters: the power to change. Nat Rev Mol Cell Biol 10:218–227
Ren C, Gu Y, Hu SY, Wu Y, Wang P, Yang YL, Yang C, Yang S, Jiang WH (2010) Identification and inactivation of pleiotropic regulator CcpA to eliminate glucose repression of xylose utilization in Clostridium acetobutylicum. Metab Eng 12(5):446–454
Ren C, Gu Y, Wu Y, Zhang WW, Yang C, Yang S, Jiang WH (2012) Pleiotropic functions of catabolite control protein CcpA in Butanol-producing Clostridium acetobutylicum. BMC Genomics 13:349
Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM (2018) Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer 18:452–464
Schiel-Bengelsdorf B, Montoya J, Linder S, Durre P (2013) Butanol fermentation. Environ Technol 34(13–16):1691–1710
Schwarz KM, Kuit W, Grimmler C, Ehrenreich A, Kengen SW (2012) A transcriptional study of acidogenic chemostat cells of Clostridium acetobutylicum—cellular behavior in adaptation to n-butanol. J Biotechnol 161(3):366–377
Shao L, Hu SY, Yang Y, Gu Y, Chen J, Yang YL, Jiang WH, Yang S (2007) Targeted gene disruption by use of a group II intron (targetron) vector in Clostridium acetobutylicum. Cell Res 17(11):963–965
Skerker JM, Prasol MS, Perchuk BS, Biondi EG, Laub MT (2005) Two-component signal transduction pathways regulating growth and cell cycle progression in a bacterium: a system-level analysis. PLoS Biol 3(10):e334
Tomas CA, Beamish J, Papoutsakis ET (2004) Transcriptional analysis of butanol stress and tolerance in Clostridium acetobutylicum. J Bacteriol 186:2006–2018
Trowitzsch S, Tampe R (2018) ABC transporters in dynamic macromolecular assemblies. J Mol Biol 430:4481–4495
Ventura JR, Hu H, Jahng D (2013) Enhanced butanol production in Clostridium acetobutylicum ATCC 824 by double overexpression of 6-phosphofructokinase and pyruvate kinase genes. Appl Microbiol Biotechnol 97(16):7505–7516
Vollherbst-Schneck K, Sands JA, Montenecourt BS (1984) Effect of butanol on lipid composition and fluidity of Clostridium acetobutylicum ATCC 824. Appl Environ Microbiol 47:193–194
Wang YF, Tian J, Ji ZH, Song MY, Li H (2016) Intracellular metabolic changes of Clostridium acetobutylicum and promotion to butanol tolerance during biobutanol fermentation. Int J Biochem Cell Biol 78:297–306
Wiesenborn DP, Rudolph FB, Papoutsakis ET (1988) Thiolase from Clostridium acetobutylicum ATCC 824 and its role in the synthesis of acids and solvents. Appl Environ Microbiol 54(11):2717–2722
Wu Y, Yang YP, Ren C, Yang C, Yang S, Gu Y, Jiang WH (2015) Molecular modulation of pleiotropic regulator CcpA for glucose and xylose coutilization by solvent-producing Clostridium acetobutylicum. Metab Eng 28:169–179
Wu YD, Xue C, Chen LJ, Yuan WJ, Bai FW (2016) Synergistic effect of calcium and zinc on glucose/xylose utilization and butanol tolerance of Clostridium acetobutylicum. FEMS Microbiol Lett 363(5):fnw023
Xiao H, Gu Y, Ning YY, Yang YL, Mitchell WJ, Jiang WH, Yang S (2011) Confirmation and elimination of xylose metabolism bottlenecks in glucose phosphoenolpyruvate-dependent phosphotransferase system-deficient Clostridium acetobutylicum for simultaneous utilization of glucose, xylose, and arabinose. Appl Environ Microbiol 77(22):7886–7895
Xu MM, Zhao JB, Yu L, Tang IC, Xue C, Yang ST (2015) Engineering Clostridium acetobutylicum with a histidine kinase knockout for enhanced n-butanol tolerance and production. Appl Microbiol Biotechnol 99(2):1011–1022
Xu MM, Zhao JB, Yu L, Yang ST (2017) Comparative genomic analysis of Clostridium acetobutylicum for understanding the mutations contributing to enhanced butanol tolerance and production. J Biotechnol 263:36–44
Xue Q, Yang YP, Chen J, Chen L, Yang S, Jiang WH, Gu Y (2016) Roles of three AbrBs in regulating two-phase Clostridium acetobutylicum fermentation. Appl Microbiol Biotechnol 100(21):9081–9089
Zhang L, Liu YQ, Yang YP, Jiang WH, Gu Y (2018) A novel dual-cre motif enables two-way autoregulation of CcpA in Clostridium acetobutylicum. Appl Environ Microbiol 84(8):e00114-18
Funding
This work was supported by the National Natural Science Foundation of China (31630003 and 31921006), and Open Funding Project of the State Key Laboratory of Bioreactor Engineering.
Author information
Authors and Affiliations
Contributions
W. J. and Y. G. designed the research; Y. Y., N. L., and L. Z. performed the research; H. W. analyzed the data; and Y.Y., W. J., and Y. G. wrote the paper.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest. This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 328 kb)
Rights and permissions
About this article
Cite this article
Yang, Y., Lang, N., Zhang, L. et al. A novel regulatory pathway consisting of a two-component system and an ABC-type transporter contributes to butanol tolerance in Clostridium acetobutylicum. Appl Microbiol Biotechnol 104, 5011–5023 (2020). https://doi.org/10.1007/s00253-020-10555-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-020-10555-6