Abstract
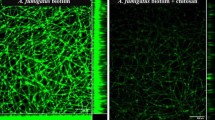
A biofilm is represented by a community of microorganisms capable of adhering to a surface and producing substances that envelop the cells, forming an extracellular matrix. The extracellular matrix is responsible for protecting microorganisms against environmental stress, hosts the immune system and confers resistance to antimicrobials. Fusarium keratoplasticum is a common species of FSSC (Fusarium solani species complex) associated with human infections, being the most prevalent species related to biofilm formation in hospital water systems and internal pipelines. With this in mind, this study aimed to characterise the biofilm formed by the fungus F. keratoplasticum and to evaluate the effects of farnesol, a fungal quorum sensing (QS) molecule, on the preformed biofilm and also during its formation at different times (adhesion and 24, 48 and 72 h). F. keratoplasticum is able to adhere to an abiotic surface and form a dense biofilm in 72 h, with increased total biomass and matrix modulation with the presence of extracellular DNA, RNA, polysaccharides and proteins. Farnesol exhibited important anti-biofilm activity, causing the destruction of hyphae and the extracellular matrix in preformed biofilm and preventing the adhesion of conidia, filamentation and the formation of biofilm. Few studies have characterised the formation of biofilm by filamentous fungi. Our findings suggest that farnesol acts efficiently on F. keratoplasticum biofilm since this molecule is capable of breaking the extracellular matrix, thereby disarranging the biofilm.





Similar content being viewed by others
References
Abdel-Rhman SH, El-Mahdy AM, El-Mowafy M (2015) Effect of tyrosol and farnesol on virulence and antibiotic resistance of clinical isolates of Pseudomonas aeruginosa. BioMed Res Int 2015:456463
Al-Hatmi AM, Meletiadis J, Curfs-Breuker I, Bonifaz A, Meis JF, De Hoog GS (2016a) In vitro combinations of natamycin with voriconazole, itraconazole and micafungin against clinical Fusarium strains causing keratitis. J Antimicrob Chemother 71:953–955
Al-Hatmi AM, Hagen F, Menken SB, Meis JF, de Hoog GS (2016b) Global molecular epidemiology and genetic diversity of Fusarium, a significant emerging group of human opportunists from 1958 to 2015. Emerg Microbes Infect 5(12):e124
Allison DL, Willems HM, Jayatilake JA, Bruno VM, Peters BM, Shirtliff ME (2016) Candida-bacteria interactions: their impact on human disease. Microbiol Spectr 4
Camarillo-Márquez O, Córdova-Alcántara IM, Hernández-Rodríguez CH, García-Pérez BE, Martínez-Rivera MA, Rodríguez-Tovar AV (2018) Antagonistic interaction of Staphylococcus aureus toward Candida glabrata during in vitro biofilm formation is caused by an apoptotic mechanism. Front Microbiol 9:2031
Chávez-Andrade GM, Tanomaru-Filho M, Rodrigues EM, Gomes-Cornélio AL, Faria G, Bernardi MIB, Guerreiro-Tanomaru JM (2017) Cytotoxicity, genotoxicity and antibacterial activity of poly (vinyl alcohol)-coated silver nanoparticles and farnesol as irrigating solutions. Arch Oral Biol 84:89–93
Clinical and Laboratory Standards Institute (2008) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard-second edition, CLSI document M38-A2. CLSI, Wayne, PA, EUA.
Clinical and Laboratory Standards Institute (2012) Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard–second edition, CLSI document M27-S4. CLSI, Wayne, PA, USA.
Cordeiro RA, Teixeira CE, Brilhante RS, Castelo-Branco DS, Paiva MA, Giffoni Leite JJ, Lima DT, Monteiro AJ, Sidrim JJ, Rocha MF (2013) Minimum inhibitory concentrations of amphotericin B, azoles and caspofungin against Candida species are reduced by farnesol. Med Mycol 51(1):53–59
de Salas F, Martínez MJ, Barriuso J (2015) Quorum-sensing mechanisms mediated by farnesol in Ophiostoma piceae: effect on secretion of sterol esterase. Appl Environ Microbiol 81(13):4351–4357
Derengowski LS, De-Souza-Silva C, Braz SV, Mello-De-Sousa TM, Báo SN, Kyaw CM, Silva-Pereira I (2009) Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann Clin Microbiol Antimicrob 8:13
Dinamarco TM, Goldman MH, Goldman GH (2011) Farnesol-induced cell death in the filamentous fungus Aspergillus nidulans. Biochem Soc Trans 39(5):1544–1548
Fairn GD, MacDonald K, McCaster CRA (2007) Chemogenomic screen in Saccharomyces cerevisiae uncovers a primary role for the mitochondria in farnesol toxicity and its regulation by the Pkc1 pathway. J Biol Chem 282(7):4868–4874
Fernandes RA, Monteiro DR, Arias LS, Fernandes GL, Delbem ACB, Barbosa DB (2018) Virulence factors in Candida albicans and Streptococcus mutans biofilms mediated by farnesol. Indian J Microbiol 58(2):138–145
Galletti J, Tobaldini-Valerio FK, Silva S, Kioshima ÉS, Trierveiler-Pereira L, Bruschi M, Negri M, Estivalet Svidzinski TI (2017) Antibiofilm activity of propolis extract on Fusarium species from onychomycosis. Fut Microbiol 12(14):1311–1321
Greguš P, Vlčková H, Buchta V, Kestřanek J, Křivčíková L, Nováková L (2010) Ultra high performance liquid chromatography tandem mass spectrometry analysis of quorum-sensing molecules of Candida albicans. J Pharm Biomed Anal 53(3):674–681
Guarro J (2013) Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur J Clin Microbiol Infect Dis 32:1491–1500
Gupta AK, Foley KA (2018) Evidence for biofilms in onychomycosis. G Ital Dermatol Venereol 154(1):50–55
Gupta C, Jongman M, Das SM, Snehaa K, Bhattacharya SN, Seyedmousavi S (2016) Genotyping and in vitro antifungal susceptibility testing of Fusarium isolates from onychomycosis in India. Mycophatologia 181(7–8):497–504
Herkert PF, Al-Hatmi A, de Oliveira Salvador GL, Muro MD, Pinheiro RL, Nucci M, Queiroz-Telles F, de Hoog GS, Meis JF (2019) Molecular characterization and antifungal susceptibility of clinical Fusarium species from Brazil. Front Microbiol 10:737
Hornby JM, Jensen EC, Lisec AD, Tasto JJ, Jahnke B, Shoemaker R, Dussault P, Nickerson KW (2001) Quorum sensing in the dimorphic fungus Candida albicans is mediated by farnesol. Appl Environ Microbiol 67(7):2982–2992
Katragkou A, McCarthy M, Alexander EL, Antachopoulos C, Meletiadis J, Jabra-Rizk MA, Petraitis V, Roilides E, Walsh TJ (2014) In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. J Antimicrob Chemother 70(2):470–478
Kirchhoff L, Olsowski M, Zilmans K, Dittmer S, Haase G, Sedlacek L, Steinmann E, Buer J, Rath P-M, Steinmann J (2017) Biofilm formation of the black yeast-like fungus Exophiala dermatitidis and its susceptibility to anti-infective agents. Sci Rep 7:42886
Koo H, Hayacibara MF, Schobel BD, Cury JA, Rosalen PL, Park YK, Vacca-Smith AM, Bowen WH (2003) Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. J Antimicrob Chemother 52(5):782–789
Krzyściak W, Kościelniak D, Papież M, Vyhouskaya P, Zagórska-Świeży K, Kołodziej I, Bystrowska B, Jurczak A (2017) Effect of a Lactobacillus salivarius probiotic on a double-species Streptococcus Mutans and Candida Albicans caries biofilm. Nutrients 9(11):1242
Leonhardt I, Spielberg S, Weber M, Albrecht-Eckardt D, Bläss M, Claus R, Barz D, Scherlach K, Hertweck C, Löffler J, Hünniger K, Kurzai O (2015) The fungal quorum-sensing molecule farnesol activates innate immune cells but suppresses cellular adaptive immunity. mBio 6(2):e00143
Liu P, Luo L, Guo J, Liu H, Wang B, Deng B, Long CA, Cheng Y (2010) Farnesol induces apoptosis and oxidative stress in the fungal pathogen Penicillium expansum. Mycologia 102(2):311–318
Lorek J, Pöggeler S, Weide MR, Breves R, Bockmühl DP (2008) Influence of farnesol on the morphogenesis of Aspergillus niger. J Basic Microbiol 48(2):99–103
Muhammed M, Anagnostou T, Desalermos A, Kourkoumpetis TK, Carneiro HA, Glavis-Bloom J, Coleman JJ, Mylonakis E (2013) Fusarium infection: report of 26 cases and review of 97 cases from the literature. Medicine 92(6):305–316
Navarathna DH, Nickerson KW, Duhamel GE, Jerrels TR, Petro TM (2007) Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. Infect Immun 75(8):4006–4011
Negri M, Silva S, Henriques M, Azeredo J, Svidzinski TIE, Oliveira R (2011) Candida tropicalis biofilms: artificial urine, urinary catheters and flow model. Med Mycol 49(7):739–747
Oliveira LT, Lopes LG, Ramos SB, Martins CHG, Jamur MC, Pires RH (2018) Fungal biofilms in the hemodialysis environment. Microb Pathogen 123:206–212
Pierce CG, Uppuluri P, Tristan AR, Wormley FL, Mowat E, Ramage G, Lopaz-Ribot J (2008) A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc 3:1494–1500
Polke M, Leonhardt I, Kurzai O, Jacobsen ID (2018) Farnesol signalling in Candida albicans – more than just communication. Crit Rev Microbiol 44:230–243
Priegnitz B-E, Wargenau A, Brandt U, Rohde M, Dietrich S, Kwade A, Krull R, Fleissner A (2012) The role of initial spore adhesion in pellet and biofilm formation in Aspergillus niger. Fung Gen Biol 49:30–38
Rajendran R, Williams C, Lappin DF, Millington O, Martins M, Ramage G (2013) Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms. Eukaryot Cell 12(3):420–429
Ramage G, Saville SP, Wickes BL, López-Ribot JL (2002) Inhibition of Candida albicans biofilm formation by farnesol, a quorum-sensing molecule. Appl Environ Microbiol 68(11):5459–5463
Ramage G, Mowat E, Jones B, Willians C, Lopez-Ribot J (2009) Our current understanding of fungal biofilms. Crit Rev Microbiol 35:340–355
Sav H, Rafati H, Öz Y, Dalyan-Cilo B, Ener B, Mohammadi F, Ilkit M, Van Diepeningen AD, Seyedmousavi S (2018) Biofilm formation and resistance to fungicides in clinically relevant members of the fungal genus Fusarium. J Fungi (Basel) 4(1):16
Schroers H-J, Samuels GJ, Zhang N, Short DPG, Juba J, Geiser DM (2016) Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia 108:806–819
Semighini CP, Hornby JM, Dumitru R, Nickerson KW, Harris SD (2006) Farnesol-induced apoptosis in Aspergillus nidulans reveals a possible mechanism for antagonistic interactions between fungi. Mol Microbiol 59(3):753–764
Semighini CP, Murray N, Harris SD (2008) Inhibition of Fusarium graminearum growth and development by farnesol. FEMS Microbiol Lett 279(2):259–264
Sharma M, Prasad R (2011) The quorum-sensing molecule farnesol is a modulator of drug efflux mediated by ABC multidrug transporters and synergizes with drugs in Candida albicans. Antimicrob Agents Chemother 55(10):4834–4843
Short DPG, O’Donnell K, Thrane U, Nielsen KF, Zhang N, Juba JH, Geiser DM (2013) Phylogenetic relationships among members of the Fusarium solani species complex in human infections and the descriptions of F. keratoplasticum sp. nov. and F. petroliphilum stat. nov. Fung Genet Biol 53:59–70
Sonnleitner E, Romeo A, Bläsi U (2012) Small regulatory RNAs in Pseudomonas aeruginosa. RNA Biol 9(4):364–371
Tamura NK, Kira G, Patussi EV, Donatti L, Svidzinski TIE (2015) Adherence and biofilm formation of Fusarium oxysporum isolated from a corneal ulcer. Glo Adv Res J Med Medical Sci 4(1):28–34
Taylor PK, Van Kessel A, Colavita A, Hancock R, Mah TF (2017) A novel small RNA is important for biofilm formation and pathogenicity in Pseudomonas aeruginosa. PloS One 12(8):e0182582
Tobaldini-Valerio FK, Bonfim-Mendonça PS, Rosseto HC, Bruschi ML, Henriques M, Negri M, Silva S, Svidzinski TIE (2016) Propolis: a potential natural product to fight Candida species infections. Future Microbiol 11:1035–1047
Toukabri N, Corpologno S, Bougnoux M-E, El Euch D, Sadfi-Zououi N, Simonetti G (2017) In vitro biofilms and antifungal susceptibility of dermatophyte and non-dermatophyte moulds involved in foot mycosis. Mycoses 61(2):79–87
Van Diepeningen AD, de Hoog GS (2016) Challenges in Fusarium, a trans-kingdom pathogen. Mycopathologia 181:161–163
Van Diepeningen AD, Feng P, Ahmed S, Sudhadham M, Bunyaratavej S, de Hoog GS (2014) Spectrum of Fusarium infections in tropical dermatology evidenced by multilocus sequencing typing diagnostics. Mycoses 58(1):48–57
Wang X, Wang Y, Zhou Y, Wei X (2014) Farnesol induces apoptosis-like cell death in the pathogenic fungus Aspergillus flavus. Mycologia 106(5):881–888
Wang YL, Liu HF, Shi XJ, Wang Y (2018) Antiproliferative activity of farnesol in HeLa cervical cancer cells is mediated via apoptosis induction, loss of mitochondrial membrane potential (ΛΨm) and PI3K/Akt signalling pathway. J BUON 23(3):752–757
Weber K, Sohr R, Schulz B, Fleischhacker M, Ruhnke M (2008) Secretion of E,E-farnesol and biofilm formation in eight different Candida species. Antimicrob Agents Chemother 52(5):1859–1861
Wojtyczka RD, Dziedzic A, Idzik D, Kępa M, Kubina R, Kabała-Dzik A, Smoleń-Dzirba J, Stojko J, Sajewicz M, Wąsik TJ (2013) Susceptibility of Staphylococcus aureus clinical isolates to propolis extract alone or in combination with antimicrobial drugs. Molecules 18:9623–9640
Acknowledgments
We thank the Complex of Research Support Centers (COMCAP), Universidade Estadual de Maringá, for their assistance in image acquisition.
Funding
This research was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), no. 421620/2018-8.
Author information
Authors and Affiliations
Contributions
B.K. performed research, analysed data and wrote the paper. G.K.S., L.U.R.C. and A.M.P. analysed data and contributed new methods or models. T.I.E.S. and M.N. conceived or designed the study and wrote the paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kischkel, B., Souza, G.K., Chiavelli, L.U.R. et al. The ability of farnesol to prevent adhesion and disrupt Fusarium keratoplasticum biofilm. Appl Microbiol Biotechnol 104, 377–389 (2020). https://doi.org/10.1007/s00253-019-10233-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-019-10233-2