Abstract
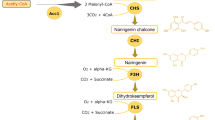
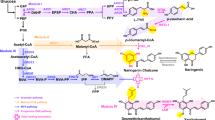
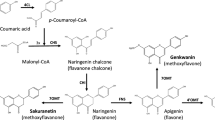
Numerous methoxylated flavonoids exhibit pronounced bioactivities. Their biotechnological production and diversification are therefore of interest to pharmaceutical and nutraceutical industries. We used a set of enzymes from sweet basil (Ocimum basilicum) to construct five strains of Saccharomyces cerevisiae producing 8- and/or 6-substituted, methoxylated flavones from their natural precursor apigenin. After identifying several growth parameters affecting the overall yields and flux, we applied optimized conditions and explored the ability of the generated strains to utilize alternative substrates. The yeast cells produced substantial amounts of 6-hydroxylated, methylated derivatives of naringenin and luteolin while the corresponding derivatives of flavonol kaempferol were only detected in trace amounts. Analysis of the intermediates and by-products of the different bioconversions suggested that the substrate specificity of both the hydroxylases and the flavonoid O-methyltransferases is imposing barriers on yields obtained with alternative substrates and highlighted steps that appear to represent bottlenecks en route to increasing the strains’ efficiencies. Additionally, analysis of flavonoid localization during fermentation revealed unequal distribution with strong intracellular accumulation of a number of methylated flavonoids and extracellular enrichment of several pathway intermediates. This work establishes a platform for the production of complex methoxylated flavonoids and discusses strategies for its improvement.







Similar content being viewed by others
References
Amor IL-B, Hehn A, Guedon E, Ghedira K, Engasser J-M, Chekir-Ghedrira L, Ghoul M (2010) Biotransformation of naringenin to eriodictyol by Saccharomyces cerevisiae functionally expressing flavonoid 3′ hydroxylase. Nat Prod Commun 5:1893–1898
Anarat-Cappillino G, Sattely ES (2014) The chemical logic of plant natural product biosynthesis. Curr Opin Plant Biol 19:51–58
Berim A, Gang DR (2013a) Characterization of two candidate flavone 8-O-methyltransferases suggests the existence of two potential routes to nevadensin in sweet basil. Phytochemistry 92:33–41
Berim A, Gang DR (2013b) The roles of a flavone 6-hydroxylase and 7-O-demethylation in the flavone biosynthetic network of sweet basil. J Biol Chem 288:1795–1805
Berim A, Gang DR (2016) Methoxylated flavones: occurrence, importance, biosynthesis. Phytochem Rev 15:363–390
Berim A, Hyatt DC, Gang DR (2012) A set of regioselective O-methyltransferases gives rise to the complex pattern of methoxylated flavones in sweet basil. Plant Physiol 160:1052–1069
Berim A, Park JJ, Gang DR (2014) Unexpected roles for ancient proteins: flavone 8-hydroxylase in sweet basil trichomes is a Rieske-type, PAO-family oxygenase. Plant J 80:385–395
Brazier-Hicks M, Edwards R (2013) Metabolic engineering of the flavone-C-glycoside pathway using polyprotein technology. Metab Eng 16:11–20
Chemler JA, Yan YJ, Leonard E, Koffas MAG (2007) Combinatorial mutasynthesis of flavonoid analogues from acrylic acids in microorganisms. Org Lett 9:1855–1858
Choi K-Y, T-j K, Koh S-K, Roh C-H, Pandey BP, Lee N, Kim B-G (2009) A-ring ortho-specific monohydroxylation of daidzein by cytochrome P450s of Nocardia farcinica IFM10152. Biotechnol J 4:1586–1595
Chouhan S, Sharma K, Zha J, Guleria S, Koffas MAG (2017) Recent advances in the recombinant biosynthesis of polyphenols. Front Microbiol 8
Conseil G, Decottignies A, Jault JM, Comte G, Barron D, Goffeau A, Di Pietro A (2000) Prenyl-flavonoids as potent inhibitors of the Pdr5p multidrug ABC transporter from Saccharomyces cerevisiae. Biochemistry 39:6910–6917
Cress BF, Leitz QD, Kim DC, Amore TD, Suzuki JY, Linhardt RJ, Koffas MAG (2017) CRISPRi-mediated metabolic engineering of E. coli for O-methylated anthocyanin production. Microb Cell Factories 16:10. https://doi.org/10.1186/s12934-016-0623-3
Eichenberger M, Lehka BJ, Folly C, Fischer D, Martens S, Simon E, Naesby M (2017) Metabolic engineering of Saccharomyces cerevisiae for de novo production of dihydrochalcones with known antioxidant, antidiabetic, and sweet tasting properties. Metab Eng 39:80–89
Ernst R, Kueppers P, Stindt J, Kuchler K, Schmitt L (2010) Multidrug efflux pumps: substrate selection in ATP-binding cassette multidrug efflux pumps—first come, first served? FEBS J 277:540–549
Eudes A, Benites VT, Wang G, Baidoo EEK, Lee TS, Keasling JD, Loque D (2015) Precursor-directed combinatorial biosynthesis of cinnamoyl, dihydrocinnamoyl, and benzoyl anthranilates in Saccharomyces cerevisiae. PLoS One 10:e0138972. https://doi.org/10.1371/journal.pone.0138972
Grayer RJ, Bryan SE, Veitch NC, Goldstone FJ, Paton A, Wollenweber E (1996) External flavones in sweet basil, Ocimum basilicum, and related taxa. Phytochemistry 43:1041–1047
Grayer RJ, Veitch NC, Kite GC, Price AM, Kokubun T (2001) Distribution of 8-oxygenated leaf-surface flavones in the genus Ocimum. Phytochemistry 56:559–567
Hovland P, Flick J, Johnston M, Sclafani RA (1989) Galactose as gratuitous inducer of GAL gene expression in yeasts growing on glucose. Gene 83:57–64
Jeon YM, Kim BG, Ahn JH (2009) Biological synthesis of 7-O-methyl apigenin from naringenin using Escherichia coli expressing two genes. J Microbiol Biotechnol 19:491–494
Jia X, Liu C, Song H, Ding M, Du J, Ma Q, Yuan Y (2016) Design, analysis and application of synthetic microbial consortia. Synth Syst Biotechnol 1:109–117
Jiang HX, Morgan JA (2004) Optimization of an in vivo plant P450 monooxygenase system in Saccharomyces cerevisiae. Biotechnol Bioeng 85:130–137
Jiang HX, Wood KV, Morgan JA (2005) Metabolic engineering of the phenylpropanoid pathway in Saccharomyces cerevisiae. Appl Environ Microbiol 71:2962–2969
Johns NI, Blazejewski T, Gomes ALC, Wang HH (2016) Principles for designing synthetic microbial communities. Curr Opin Microbiol 31:146–153
Jones JA, Toparlak OD, Koffas MAG (2015) Metabolic pathway balancing and its role in the production of biofuels and chemicals. Curr Opin Biotechnol 33:52–59
Jones JA, Vernacchio VR, Collins SM, Shirke AN, Xiu Y, Englaender JA, Cress BF, McCutcheon CC, Linhardt RJ, Gross RA, Koffas MAG (2017) Complete biosynthesis of anthocyanins using E. coli polycultures. MBio 8:e00621–e00617. https://doi.org/10.1128/mBio.00621-17
Kim DH, Kim BG, Lee Y, Ryu JY, Lim Y, Hur HG, Ahn JH (2005) Regiospecific methylation of naringenin to ponciretin by soybean O-methyltransferase expressed in Escherichia coli. J Biotechnol 119:155–162
Kim BG, Jung BR, Lee Y, Hur HG, Lim Y, Ahn JH (2006a) Regiospecific flavonoid 7-O-methylation with Streptomyces avermitilis O-methyltransferase expressed in Escherichia coli. J Agric Food Chem 54:823–828
Kim JH, Kim BG, Park Y, Han JH, Lim Y, Ahn J-H (2006b) Production of 5,7-dihydroxy, 3′,4′,5′-trimethoxyflavone from 5,7,3′,4′,5′-pentahydroxyflavone using two O-methyltransferases expressed in Escherichia coli. Agric Chem Biotechnol 49:114–116
Kim B-G, Lee YJ, Lee S, Lim Y, Cheong Y, Ahn J-H (2008) Altered regioselectivity of a poplar O-methyltransferase, POMT-7. J Biotechnol 138:107–111
Koirala N, Pandey RP, Parajuli P, Jung HJ, Sohng JK (2014) Methylation and subsequent glycosylation of 7,8-dihydroxyflavone. J Biotechnol 184:128–137
Koirala N, Thuan NH, Ghimire GP, Thang DV, Sohng JK (2016) Methylation of flavonoids: chemical structures, bioactivities, progress and perspectives for biotechnological production. Enzym Microb Technol 86:103–116
Koopman F, Beekwilder J, Crimi B, van Houwelingen A, Hall RD, Bosch D, van Maris AJA, Pronk JT, Daran J-M (2012) De novo production of the flavonoid naringenin in engineered Saccharomyces cerevisiae. Microb Cell Factories 11:155. https://doi.org/10.1186/1475-2859-11-155
Latunde-Dada AO, Cabello-Hurtado F, Czittrich N, Didierjean L, Schopfer C, Hertkorn N, Werck-Reichhart D, Ebel J (2001) Flavonoid 6-hydroxylase from soybean (Glycine max L.), a novel plant P-450 monooxygenase. J Biol Chem 276:1688–1695
Lee H, Kim BG, Ahn JH (2014) Production of bioactive hydroxyflavones by using monooxygenase from Saccharothrix espanaensis. J Biotechnol 176:11–17
Lee H, Kim BG, Kim M, Ahn JH (2015) Biosynthesis of two flavones, apigenin and genkwanin, in Escherichia coli. J Microbiol Biotechnol 25:1442–1448
Lee D, Park HL, Lee SW, Bhoo SH, Cho MH (2017) Biotechnological production of dimethoxyflavonoids using a fusion flavonoid O-methyltransferase possessing both 3′- and 7-O-methyltransferase activities. J Nat Prod 80:1467–1474
Leonard E, Yan YJ, Lim KH, Koffas MAG (2005) Investigation of two distinct flavone synthases for plant-specific flavone biosynthesis in Saccharomyces cerevisiae. Appl Environ Microbiol 71:8241–8248
Leonard E, Chemler J, Lim KH, Koffas MAG (2006a) Expression of a soluble flavone synthase allows the biosynthesis of phytoestrogen derivatives in Escherichia coli. Appl Microbiol Biotechnol 70:85–91
Leonard E, Yan YJ, Koffas MAG (2006b) Functional expression of a P450 flavonoid hydroxylase for the biosynthesis of plant-specific hydroxylated flavonols in Escherichia coli. Metab Eng 8:172–181
Lim CG, Wong L, Bhan N, Dvora H, Xu P, Venkiteswaran S, Koffas MAG (2015) Development of a recombinant Escherichia coli strain for overproduction of the plant pigment anthocyanin. Appl Environ Microbiol 81:6276–6284
Malla S, Koffas MAG, Kazlauskas RJ, Kim BG (2012) Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl Environ Microbiol 78:684–694
Pandey RP, Parajuli P, Koffas MAG, Sohng JK (2016) Microbial production of natural and non-natural flavonoids: pathway engineering, directed evolution and systems/synthetic biology. Biotechnol Adv 34:634–662
Rodriguez A, Strucko T, Stahlhut SG, Kristensen M, Svenssen DK, Forster J, Nielsen J, Borodina I (2017) Metabolic engineering of yeast for fermentative production of flavonoids. Bioresour Technol 245:1645–1654
Rogers B, Decottignies A, Kolaczkowski M, Carvajal E, Balzi E, Goffeau A (2001) The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J Mol Microbiol Biotechnol 3:207–214
Santos CNS, Koffas M, Stephanopoulos G (2011) Optimization of a heterologous pathway for the production of flavonoids from glucose. Metab Eng 13:392–400
Schmidt A, Li C, Shi F, Jones AD, Pichersky E (2011) Polymethylated myricetin in trichomes of the wild tomato species Solanum habrochaites and characterization of trichome-specific 3 '/5 '- and 7/4 '-myricetin O-methyltransferases. Plant Physiol 155:1999–2009
Shimizu T, Lin F, Hasegawa M, Okada K, Nojiri H, Yamane H (2012) Purification and identification of naringenin 7-O-methyltransferase, a key enzyme in biosynthesis of flavonoid phytoalexin sakuranetin in rice. J Biol Chem 287:19315–19325
Sordon S, Madej A, Poplonski J, Bartmanska A, Tronina T, Brzezowska E, Juszczyk P, Huszcza E (2016) Regioselective ortho-hydroxylations of flavonoids by yeast. J Agric Food Chem 64:5525–5530
Trantas E, Panopoulos N, Ververidis F (2009) Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng 11:355–366
Trantas EA, Koffas MAG, Xu P, Ververidis F (2015) When plants produce not enough or at all: metabolic engineering of flavonoids in microbial hosts. Front Plant Sci 6. https://doi.org/10.3389/fpls.2015.00007
Ververidis F, Trantas E, Douglas C, Vollmer G, Kretzschmar G, Panopoulos N (2007) Biotechnology of flavonoids and other phenylpropanoid-derived natural products. Part I: chemical diversity, impacts on plant biology and human health. Biotechnol J 2:1214–1234
Wang Y, Halls C, Zhang J, Matsuno M, Zhang Y, Yu O (2011) Stepwise increase of resveratrol biosynthesis in yeast Saccharomyces cerevisiae by metabolic engineering. Metab Eng 13:455–463
Werner SR, Morgan JA (2009) Expression of a Dianthus flavonoid glucosyltransferase in Saccharomyces cerevisiae for whole-cell biocatalysis. J Biotechnol 142:233–241
Williams IS, Chib S, Nuthakki VK, Gatchie L, Joshi P, Narkhede NA, Vishwakarma RA, Bharate SB, Saran S, Chaudhuri B (2017) Biotransformation of chrysin to baicalein: selective C6-hydroxylation of 5,7-dihydroxyflavone using whole yeast cells stably expressing human CYP1A1 enzyme. J Agric Food Chem 65:7440–7446
Willits MG, Giovanni M, Prata RTN, Kramer CM, De Luca V, Steffens JC, Graser G (2004) Bio-fermentation of modified flavonoids: an example of in vivo diversification of secondary metabolites. Phytochemistry 65:31–41
Yan YJ, Kohli A, Koffas MAG (2005) Biosynthesis of natural flavanones in Saccharomyces cerevisiae. Appl Environ Microbiol 71:5610–5613
Yan YJ, Li Z, Koffas MAG (2008) High-yield anthocyanin biosynthesis in engineered Escherichia coli. Biotechnol Bioeng 100:126–140
Zhang H, Wang X (2016) Modular co-culture engineering, a new approach for metabolic engineering. Metab Eng 37:114–121
Zhao Q, Cui M-Y, Levsh O, Yang D, Liu J, Li J, Hill L, Yang L, Hu Y, Weng J-K, Chen X-Y, Martin C (2018) Two CYP82D enzymes function as flavone hydroxylases in the biosynthesis of root-specific 4′-deoxyflavones in Scutellaria baicalensis. Mol Plant 11:135–148
Zhou K, Qiao KJ, Edgar S, Stephanopoulos G (2015) Distributing a metabolic pathway among a microbial consortium enhances production of natural products. Nat Biotechnol 33:377–383
Acknowledgements
AB would like to thank Dr. G. O. Berim (SUNY at Buffalo, NY) for help with statistical analysis.
Funding
This work was in part supported by the US Department of Energy Biological and Environmental Research Program (grant DE-SC0001728 to D.R.G.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals
Electronic supplementary material
ESM 1
(PDF 948 kb)
Rights and permissions
About this article
Cite this article
Berim, A., Gang, D.R. Production of methoxylated flavonoids in yeast using ring A hydroxylases and flavonoid O-methyltransferases from sweet basil. Appl Microbiol Biotechnol 102, 5585–5598 (2018). https://doi.org/10.1007/s00253-018-9043-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9043-0