Abstract
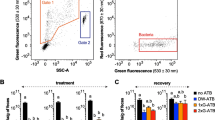
The experiment was carried out on 24 SPF BALB/c female mice and lasted for 15 days with a 5-day antibiotic (ATB) treatment and then 10 days without ATB treatment. The aim of our study was to acquire an animal model with reduced and controlled microflora and, at the same time, to ensure that the good health of these animals is maintained. Per oral administration of amoxicillin and clavulanate potassium in Amoksiklav (Sandoz, Slovenia) at a dose of 387.11 mg/kg body weight (0.2 ml of dilution per mouse) and subcutaneous administration of ciprofloxacin in Ciloxan (Alcon, Spain) at a dose of 18.87 mg/kg body weight (0.1 ml of dilution per mouse) were performed every 12 h during first 5 days of experiment. Five-day treatment with ATB led to a reduced survivability of microorganisms in faeces (28.33 ± 0.43 % on day 2) and caecum content (28.10 ± 1.56 %), where no cultivable microorganisms in faeces were present. Ten-day convalescence of decontaminated animals under gnotobiotic conditions prevented recovery of species diversity in mice gut microflora. This was reduced to two detectable cultivable species, namely Escherichia coli (GenBank KX086704) and Enterococcus sp. (GenBank KX086705) which were capable to restore its metabolic (CRL 2012) and morphological potential (Baratta et al. Histochem Cell Biol 131:713–726, 2009) within physiological range. Animals obtained under this procedure can be used in further studies. As a result, we created a mouse gnoto model with reduced and controlled microflora without alteration of the overall health status of the respective animals.




Similar content being viewed by others
References
Al-Asmakh M, Anuar F, Zadjali F, Rafter J, Pettersson S (2012) Gut microbial communities modulating brain development and function. Gut Microbes 3:366–373
Al-Asmakh M, Stukenborg JB, Reda A, Anuar F, Strand ML, Hedin L, Pettersson S, Soeder O (2014) The gut microbiota and developmental programming of the testis in mice PLoS One 9 doi:10.1371/journal.pone.0103809
Antonopoulos DA, Huse SM, Morrison HG, Schmidt TM, Sogin ML, Young VB (2009) Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun 77:2367–2375. doi:10.1128/iai.01520-08
Baratta JL, Ngo A, Lopez B, Kasabwalla N, Longmuir KJ, Robertson RT (2009) Cellular organization of normal mouse liver: a histological, quantitative immunocytochemical, and fine structural analysis. Histochem Cell Biol 131:713–726. doi:10.1007/s00418-009-0577-1
Bergan T, Delin C, Johansen S, Kolstad IM, Nord CE, Thorsteinsson SB (1986) Pharmacokinetics of ciprofloxacin and effect of repeated dosage on salivary and fecal microflora. Antimicrob Agents Chemother 29:298–302
Bleich A, Hansen AK (2012) Time to include the gut microbiota in the hygienic standardisation of laboratory rodents. Comp Immunol Microbiol Infect Dis 35:81–92. doi:10.1016/j.cimid.2011.12.006
Charles River Laboratories International (2012) BALB/C Mouse Hematology and Biochemistry North American Colonies January 2008 – December 2012 http://www.criver.com/files/pdfs/rms/balbc/rm_rm_r_balb-c_mouse_clinical_pathology_data.aspx
Chen L, Zhan C, Wang L (2015) Jin Y-l, Shi Y, Wang Q. Biological characteristics of germ free animals and its application in the research on human diseases Fu Dan Xue Bao Zi Ran Ke Xue Ban 42:409–412. doi:10.3969/j.issn.1672-8467.2015.03.022
Deoca J, Millat E, Dominguez MA, Aldeano A, Martin R (1993) Selective bowel decontamination, nutritional therapy and bacterial translocation after burn injury. Clin Nutr 12:355–359. doi:10.1016/0261-5614(93)90032-y
Donskey CJ, Hanrahan JA, Hutton RA, Rice LB (1999) Effect of parenteral antibiotic administration on persistence of vancomycin-resistant Enterococcus faecium in the mouse gastrointestinal tract. J Infect Dis 180:384–390. doi:10.1086/314874
E.O.R.T.C (1982) Gnotobiotic project group: a prospective cooperative study of antimicrobial decontamination in granulocytopenic patients. Comparison of two different methods. Infection 10:131–138
Gancarčíková S (2012) Differences in the development of the small intestine between gnotobiotic and conventionally bred piglets. In: Brzozowski T (ed) New advances in the basic and clinical gastroenterology. InTech, Rijeka, pp. 375–414
Gosney M, Martin MV, Wright AE (2006) The role of selective decontamination of the digestive tract in acute stroke. Age Ageing 35:42–47. doi:10.1093/ageing/afj019
Grasa L, Abecia L, Forcen R, Castro M, de Jalon Garcia JA, Latorre E, Alcalde AI, Murillo MD (2015) Antibiotic-induced depletion of murine microbiota induces mild inflammation and changes in toll-like receptor patterns and intestinal motility. Microb Ecol 70:835–848. doi:10.1007/s00248-015-0613-8
Guo WD, Ding JY, Huang QH, Jerrells T, Deitch EA (1995) Alterations in intestinal bacterial flora modulate the systemic cytokine response to hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol 269:G827–G832
Hansen AK, Velschow S (2000) Antibiotic resistance in bacterial isolates from laboratory animal colonies naive to antibiotic treatment. Lab Anim 34:413–422
Hayes NR, van der Waaij D, Cohen BJ (1974) Elimination of bacteria from dogs with antibiotics. J Hyg 73:205–212
Herrine SK, Rossi S, Navarro VJ (2006) Management of patients with chronic hepatitis C infection. Clin Exp Med 6:20–26. doi:10.1007/s10238-006-0089-4
Hoy YE, Bik EM, Lawley TD, Holmes SP, Monack DM, Theriot JA, Relman DA (2015) Variation in taxonomic composition of the fecal microbiota in an inbred mouse strain across individuals and time PLoS One 10 doi:10.1371/journal.pone.0142825
Johnson SA, Nicolson SW, Jackson S (2004) The effect of different oral antibiotics on the gastrointestinal microflora of a wild rodent (Aethomys namaquensis). Comp Biochem Physiol A Mol Integr Physiol 138:475–483. doi:10.1016/j.cbpb.2004.06.010
Luiten EJT, Bruining HA (1999) Antimicrobial prophylaxis in acute pancreatitis: selective decontamination versus antibiotics. Best Pract Res Clin Gastroenterol 13:317–330. doi:10.1053/bega.1999.0027
Mandel L, Talafantova M, Trebichavsky I, Travnicek J, Koukal M (1985) Selective decontamination, induced colonization resistance and connected immunological changes in piglets. Folia Microbiol 30:312–318. doi:10.1007/bf02923525
Mudronova D (2015) Flow cytometry as an auxiliary tool for the selection of probiotic bacteria. Benef Microbes 6:727–734. doi:10.3920/bm2014.0145
Naef F, Warschkow R, Kolb W, Zuend M, Lange J, Steffen T (2010) Selective decontamination of the gastrointestinal tract in patients undergoing esophageal resection. Bmc Surg 10 doi:10.1186/1471–2482–10-36
Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S (2012) Host-gut microbiota metabolic interactions. Science 336:1262–1267. doi:10.1126/science.1223813
Perez F, Pultz MJ, Endimiani A, Bonomo RA, Donskey CJ (2011) Effect of antibiotic treatment on establishment and elimination of intestinal colonization by KPC-producing Klebsiella pneumoniae in mice. Antimicrob Agents Chemother 55:2585–2589. doi:10.1128/aac.00891-10
Puhl NJ, Uwiera RRE, Yanke LJ, Selinger LB, Inglis GD (2012) Antibiotics conspicuously affect community profiles and richness, but not the density of bacterial cells associated with mucosa in the large and small intestines of mice. Anaerobe 18:67–75. doi:10.1016/j.anaerobe.2011.12.007
Ramounaupigot A, Monnet P, Boyer G, Darbas H, Donadio D (1991) Prevention of septicemia caused by gastrointestinal-tract organisms in patients with granulopenia—comparison of 3 gastrointestinal-tract decontamination regimens. Pathol Biol 39:131–135
Schlegel L, Coudray-Lucas C, Barbut F, Le Boucher J, Jardel A, Zarrabian S, Cynober L (2000) Bacterial dissemination and metabolic changes in rats induced by endotoxemia following intestinal E-coli overgrowth are reduced by ornithine alpha-ketoglutarate administration. J Nutr 130:2897–2902
Shimizu T, Tani T, Hanasawa K, Endo Y, Kodama M (2001) The role of bacterial translocation on neutrophil activation during hemorrhagic shock in rats. Shock 16:59–63. doi:10.1097/00024382-200116010-00011
Silvestri L, van Saene HKF, Milanese M, Gregori D, Gullo A (2007) Selective decontamination of the digestive tract reduces bacterial bloodstream infection and mortality in critically ill patients. Systematic review of randomized, controlled trials. J Hosp Infect 65:187–203. doi:10.1016/j.jhin.2006.10.014
Sjogren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Backhed F, Ohlsson C (2012) The gut microbiota regulates bone mass in mice. J Bone Miner Res 27:1357–1367. doi:10.1002/jbmr.1588
Srivastava KK (1988) Elimination of microbial-flora from conventionally raised Syrian-hamsters by antimicrobial agents. Lab Anim Sci 38:169–172
Stecher B, Robbiani R, Walker AW, Westendorf AM, Barthel M, Kremer M, Chaffron S, Macpherson AJ, Buer J, Parkhill J, Dougan G, von Mering C, Hardt WD (2007) Salmonella enterica serovar typhimurium exploits inflammation to compete with the intestinal microbiota. PLoS Biol 5:2177–2189. doi:10.1371/journal.pbio.0050244
Stehr M, Greweling MC, Tischer S, Singh M, Bloecker H, Monner DA, Mueller W (2009) Charles River altered Schaedler flora (CRASF (R)) remained stable for four years in a mouse colony housed in individually ventilated cages. Lab Anim 43:362–370. doi:10.1258/la.2009.0080075
Stepankova R, Tonar Z, Bartova J, Nedorost L, Rossman P, Poledne R, Schwarzer M, Tlaskalova-Hogenova H (2010) Absence of microbiota (germ-free conditions) accelerates the atherosclerosis in ApoE-deficient mice fed standard low cholesterol diet. J Atheroscler Thromb 17:796–804
Stevens A, Lowe JS, Young B (2002) Wheater’s basic histopathology: a colour atlas and text. Churchill Livingstone.
The European Committee on Antimicrobial Susceptibility Testing (2016) Breakpoint tables for interpretation of MICs and zone diameters Version 6.0 2016 http://www.eucast.org
The Institutional Animal Care And Use Committee (2012) Retro-orbital blood collection in mice IACUC / LARC Standard Procedures http://www.iacuc.ucsf.edu/Policies/RetroObitalBloodCollection.doc
Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MRM, Kamboj M, Pamer EG (2010) Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341. doi:10.1172/jci43918
Van der Waaij D, De Vries JM, Leekerkerk JEC (1970) Eliminating bacteria from monkeys with antibiotics in: Balner H, Beveridge W (ed) infections and immunosuppression in subhuman primates. Munksgaard, Copenhagen, pp. 21–23
Van der Waaij D, Nord CE (2000) Development and persistence of multi-resistance to antibiotics in bacteria; an analysis and a new approach to this urgent problem. Int J Antimicrob Agents 16:191–197. doi:10.1016/s0924-8579(00)00227-2
Van der Waaij D, Sturm CA (1968) Antibiotic decontimination of the digestive tract of mice. Technical procedures. Lab Anim Care 18:1–10
Van der Waaij LA, Messerschmidt O, Van der Waaij D (1989) A norfloxacin dose finding study for selective decontamination of the digestive-tract in pigs. Epidemiol Infect 102:93–103
Vossen JM, Guiot HFL, Lankester AC, Vossen ACTM, Bredius RGM, Wolterbeek R, Bakker HDJ, Heidt PJ (2014) Complete suppression of the gut microbiome prevents acute graft-versus-host disease following allogeneic bone marrow transplantation. PLoS One 9 doi:10.1371/journal.pone.0105706
Wiesner SM, Jechorek RP, Garni RM, Bendel CM, Wells CL (2001) Gastrointestinal colonization by Candida albicans mutant strains in antibiotic-treated mice. Clin Diagn Lab Immunol 8:192–195. doi:10.1128/cdli.8.1.192-195.2001
Wilson KH, Brown RS, Andersen GL, Tsang J, Sartor B (2006) Comparison of fecal biota from specific pathogen free and feral mice. Anaerobe 12:249–253. doi:10.1016/j.anaerobe.2006.09.002
Yuan J, Wei H, Zeng B, Tang H, Li W, Zhang Z (2010) Impact of neonatal antibiotic treatment on the biodiversity of the murine intestinal lactobacillus community. Curr Microbiol 60:6–11. doi:10.1007/s00284-009-9492-x
Acknowledgments
This publication was supported by the Slovak Research and Development Agency under the contract no. APVV-0199-11, by the project no. 26220220152 implementation supported by the Research & Development Operational Programme funded by the ERDF and project VEGA no. 1/0009/15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The experiment was carried out at the Department of Microbiology and Gnotobiology, University of Veterinary Medicine and Pharmacy (UVMP), Košice, Slovakia. The State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 1177/14-221, and the animals were handled and sacrificed in a humane manner in accordance with the guidelines established by the relevant commission. All applicable international, national and institutional guidelines for the care and use of animals were followed.
Funding
This study was funded by the Slovak Research and Development Agency under the contract no. APVV-0199-11, by the project no. 26220220152 implementation supported by the Research & Development Operational Programme funded by the ERDF and project VEGA no. 1/0009/15.
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
The State Veterinary and Food Administration of the Slovak Republic approved the experimental protocol number 1177/14-221, and the animals were handled and sacrificed in a humane manner in accordance with the guidelines established by the relevant commission. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Popper, M., Gancarčíková, S., Maďar, M. et al. Amoxicillin-clavulanic acid and ciprofloxacin-treated SPF mice as gnotobiotic model. Appl Microbiol Biotechnol 100, 9671–9682 (2016). https://doi.org/10.1007/s00253-016-7855-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-016-7855-3