Abstract
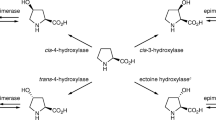
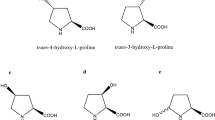
Naturally occurring l-hydroxyproline in its four regio- and stereoisomeric forms has been explored as a possible precursor for pharmaceutical agents, yet the selective synthesis of trans-3-hydroxy-l-proline has not been achieved. Our aim was to develop a novel biocatalytic asymmetric method for the synthesis of trans-3-hydroxy-l-proline. So far, we focused on the rhizobial arginine catabolic pathway: arginase and ornithine cyclodeaminase are involved in l-arginine degradation to l-proline via l-ornithine. We hypothesized that trans-3-hydroxy-l-proline should be synthesized if arginase and ornithine cyclodeaminase act on (2S,3S)-3-hydroxyarginine and (2S,3S)-3-hydroxyornithine, respectively. To test this hypothesis, we cloned the genes of l-arginine 3-hydroxylase, arginase, and ornithine cyclodeaminase and overexpressed them in Escherichia coli, with subsequent enzyme purification. After characterization and optimization of each enzyme, a three-step procedure involving l-arginine 3-hydroxylase, arginase, and ornithine cyclodeaminase (in this order) was performed using l-arginine as a starting substrate. At the second step of the procedure, putative hydroxyornithine was formed quantitatively by arginase from (2S,3S)-3-hydroxyarginine. Nuclear magnetic resonance and chiral high-performance liquid chromatography analyses revealed that the absolute configuration of this compound was (2S,3S)-3-hydroxyornithine. In the last step of the procedure, trans-3-hydroxy-l-proline was synthesized selectively by ornithine cyclodeaminase from (2S,3S)-3-hydroxyornithine. Thus, we successfully developed a novel synthetic route, comprised of three reactions, to convert l-arginine to trans-3-hydroxy-l-proline. The excellent selectivity makes this procedure simpler and more efficient than conventional chemical synthesis.






Similar content being viewed by others
References
Abdelal AT (1979) Arginine catabolism by microorganisms. Annu Rev Microbiol 33:139–168
Alam S, Wang SC, Ruzicka FJ, Frey PA, Wedekind JE (2004) Crystallization and X-ray diffraction analysis of ornithine cyclodeaminase from Pseudomonas putida. Acta Crystallogr D Biol Crystallogr 60:941–944
Baud D, Saaidi PL, Monfleur A, Harari M, Cuccaro J, Fossey A, Besnard M, Debard A, Mariage A, Pellouin V, Petit JL, Salanoubat M, Weissenbach J, de Berardinis V, Zaparucha A (2014) Synthesis of mono- and dihydroxylated amino acids with new α-ketoglutarate-dependent dioxygenases: biocatalytic oxidation of C–H bonds. ChemCatChem 6:3012–3017
Beringer JE (1974) R factor transfer in Rhizobium leguminosarum. J Gen Microbiol 84:188–198
Bewley MC, Jeffrey PD, Patchett ML, Kanyo ZF, Baker EN (1999) Crystal structures of Bacillus caldovelox arginase in complex with substrate and inhibitors reveal new insights into activation, inhibition and catalysis in the arginase superfamily. Structure 7:435–448
Bhushan R, Bruckner H (2004) Marfey’s reagent for chiral amino acid analysis: a review. Amino Acids 27:231–247
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen L, Yue Q, Zhang X, Xiang M, Wang C, Li S, Che Y, Ortiz-Lopez FJ, Bills GF, Liu X, An Z (2013) Genomics-driven discovery of the pneumocandin biosynthetic gene cluster in the fungus Glarea lozoyensis. BMC Genomics 14:339
Connors N, Petersen L, Hughes R, Saini K, Olewinski R, Salmon P (2000) Residual fructose and osmolality affect the levels of pneumocandins B0 and C0 produced by Glarea lozoyensis. Appl Microbiol Biotechnol 54:814–818
DeMong DE, Williams RM (2001) An efficient asymmetric synthesis of (2S,3S)- and (2R,3R)-β-hydroxyornithine. Tetrahedron Lett 42:183–185
Dessaux Y, Petit A, Tempe J, Demarez M, Legrain C, Wiame JM (1986) Arginine catabolism in Agrobacterium strains: role of the Ti plasmid. J Bacteriol 166:44–50
Digiovanni MC, Misiti D, Zappia G, Dellemonache G (1993) A stereoselective synthesis of 3(R)-hydroxy-2(S)-ornithine. Tetrahedron 49:11321–11328
Durand JO, Larcheveque M, Petit Y (1998) Reductive cyanation: a key step for a short synthesis of (−)-(2S,3S)-3-hydroxyproline. Tetrahedron Lett 39:5743–5746
Fujii Y, Kabumoto H, Nishimura K, Fujii T, Yanai S, Takeda K, Tamura N, Arisawa A, Tamura T (2009) Purification, characterization, and directed evolution study of a vitamin D3 hydroxylase from Pseudonocardia autotrophica. Biochem Biophys Res Commun 385:170–175
Fujiwara S, Nagai Y (1981) Bovine renal cortex type I collagen: high contents of 3- and 4-hydroxyprolines. J Biochem 89:1397–1401
Goodman JL, Wang S, Alam S, Ruzicka FJ, Frey PA, Wedekind JE (2004) Ornithine cyclodeaminase: structure, mechanism of action, and implications for the μ-crystallin family. Biochemistry 43:13883–13891
Gould SJ, Ju S (1992) Biosynthesis of acivicin. 3. Incorporation of ornithine and N δ-hydroxyornithine. J Am Chem Soc 114:10166–10172
Green MR, Sambrook J (2012) Molecular cloning: a laboratory manual, 4th edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
Hara R, Kino K (2009) Characterization of novel 2-oxoglutarate dependent dioxygenases converting l-proline to cis-4-hydroxy-l-proline. Biochem Biophys Res Commun 379:882–886
Hashizume H, Hirosawa S, Sawa R, Muraoka Y, Ikeda D, Naganawa H, Igarashi M (2004) Tripropeptins, novel antimicrobial agents produced by Lysobacter sp. II. Structure elucidation. J Antibiot (Tokyo) 57:52–58
Helmetag V, Samel SA, Thomas MG, Marahiel MA, Essen LO (2009) Structural basis for the erythro-stereospecificity of the l-arginine oxygenase VioC in viomycin biosynthesis. FEBS J 276:3669–3682
Herdeis C, Hubmann HP, Lotter H (1994) Chiral pool synthesis of trans-(2S,3S)-3-hydroxyproline and castanodiol from S-pyroglutamic acid. Tetrahedron Asymmetry 5:119–128
Houwaart S, Youssar L, Hüttel W (2014) Pneumocandin biosynthesis: involvement of a trans-selective proline hydroxylase. Chembiochem 15:2365–2369
Hughes P, Clardy J (1989) Total synthesis of 3(S)-carboxy-4(S)-hydroxy-2,3,4,5-tetrahydropyridazine, an unusual amino-acid constituent of luzopeptin-A. J Org Chem 54:3260–3264
Irreverre F, Witkop B, Robertson AV, Morita K (1962) Isolation and synthesis of 3-hydroxy-l-proline. Biochem Biophys Res Commun 8:453–455
Kim J, Mayfield JE (1997) Brucella abortus arginase and ornithine cyclodeaminase genes are similar to Ti plasmid arginase and ornithine cyclodeaminase. Biochim Biophys Acta 1354:55–57
Kite GC, Alison PC, Burke A, Simmonds MS, Blaney WM, Fellows LE (1995) Accumulation of trans-3-hydroxy-l-proline by seeds and leaves of the edible Madagascan legume Lemuropisum edule H. Perrier. Kew Bull 50:585–590
Krol WJ, Mao SS, Steele DL, Townsend CA (1991) Stereochemical correlation of proclavaminic acid and syntheses of erythro- and threo-l-β-hydroxyornithine from an improved vinylglycine synthon. J Org Chem 56:728–731
Kumar TP, Chandrasekhar S (2012) Asymmetric syntheses of all stereoisomers of 3-hydroxyproline; a constituent of several bioactive compounds. Synthesis 44:2889–2894
Lee JH, Kang JE, Yang MS, Kang KY, Park KH (2001) Efficient synthesis of 3-hydroxyprolines and 3-hydroxyprolinols from sugars. Tetrahedron 57:10071–10076
Maarsingh H, Zaagsma J, Meurs H (2009) Arginase: a key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br J Pharmacol 158:652–664
Matsuoka T, Miyakoshi S, Tanzawa K, Nakahara K, Hosobuchi M, Serizawa N (1989) Purification and characterization of cytochrome P-450sca from Streptomyces carbophilus. ML-236B (compactin) induces a cytochrome P-450sca in Streptomyces carbophilus that hydroxylates ML-236B to pravastatin sodium (CS-514), a tissue-selective inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme-A reductase. Eur J Biochem 184:707–713
Mori H, Shibasaki T, Yano K, Ozaki A (1997) Purification and cloning of a proline 3-hydroxylase, a novel enzyme which hydroxylates free l-proline to cis-3-hydroxy-l-proline. J Bacteriol 179:5677–5683
Morita K, Irreverre F, Sakiyama F, Witkop B (1963) One-step synthesis and enzymatic resolution of cis- and trans-3-hydroxyproline. J Am Chem Soc 85:2832–2834
Muth WL, Costilow RN (1974) Ornithine cyclase (Deaminating): II. Properties of the homogenous enzyme. J Biol Chem 249:7457–7462
Ogawa J, Shimizu S (1999) Microbial enzymes: new industrial applications from traditional screening methods. Trends Biotechnol 17:13–21
Pandey SK, Kumar P (2006) Enantioselective synthesis of (2R,3R)- and (2S,3S)-β-hydroxyornithine. Tetrahedron Lett 47:4167–4169
Petersen LA, Hughes DL, Hughes R, DiMichele L, Salmon P, Connors N (2001) Effects of amino acid and trace element supplementation on pneumocandin production by Glarea lozoyensis: impact on titer, analogue levels, and the identification of new analogues of pneumocandin B0. J Ind Microbiol Biotechnol 26:216–221
Petersen L, Olewinski R, Salmon P, Connors N (2003) Novel proline hydroxylase activities in the pneumocandin-producing fungus Glarea lozoyensis responsible for the formation of trans 3- and trans 4-hydroxyproline. Appl Microbiol Biotechnol 62:263–267
Ramaswamy SG (1983) Preparation of 14C-3-hydroxyproline from 14C-proline by peroxidation. J Labelled Comp Radiopharm 20:233–242
Rao BV, Krishna UM, Gurjar MK (1997) Stereoselective synthesis of [2S,3R]-β-hydroxy ornithine. Synth Commun 27:1335–1345
Ricciardolo FL, Zaagsma J, Meurs H (2005) The therapeutic potential of drugs targeting the arginase pathway in asthma. Expert Opin Investig Drugs 14:1221–1231
Sans N, Schroder G, Schroder J (1987) The Noc region of Ti plasmid C58 codes for arginase and ornithine cyclodeaminase. Eur J Biochem 167:81–87
Sans N, Schindler U, Schroder J (1988) Ornithine cyclodeaminase from Ti plasmid C58: DNA sequence, enzyme properties and regulation of activity by arginine. Eur J Biochem 173:123–130
Schindler U, Sans N, Schroder J (1989) Ornithine cyclodeaminase from octopine Ti plasmid Ach5: identification, DNA sequence, enzyme properties, and comparison with gene and enzyme from nopaline Ti plasmid C58. J Bacteriol 171:847–854
Schwartz RE, Sesin DF, Joshua H, Wilson KE, Kempf AJ, Goklen KA, Kuehner D, Gailliot P, Gleason C, White R, Inamine E, Bills G, Salmon P, Zitano L (1992) Pneumocandins from Zalerion arboricola. I. Discovery and isolation. J Antibiot (Tokyo) 45:1853–1866
Sheehan JC, Mania D, Nakamura S, Stock JA, Maeda K (1968) The structure of telomycin. J Am Chem Soc 90:462–470
Shibasaki T, Mori H, Chiba S, Ozaki A (1999) Microbial proline 4-hydroxylase screening and gene cloning. Appl Environ Microbiol 65:4028–4031
Shibasaki T, Mori H, Ozaki A (2000) Enzymatic production of trans-4-hydroxy-l-proline by regio- and stereospecific hydroxylation of l-proline. Biosci Biotechnol Biochem 64:746–750
Sinha S, Tilve S, Chandrasekaran S (2005) A convenient synthesis of trans-3-hydroxy-l-proline. Arkivoc 209–217
Soto MJ, Vandillewijn P, Olivares J, Toro N (1994) Ornithine cyclodeaminase activity in Rhizobium meliloti. FEMS Microbiol Lett 119:209–213
Suksamrarn S, Suwannapoch N, Aunchai N, Kuno M, Ratananukul P, Haritakun R, Jansakul C, Ruchirawat S (2005) Ziziphine N, O, P and Q, new antiplasmodial cyclopeptide alkaloids from Ziziphus oenoplia var. brunoniana. Tetrahedron 61:1175–1180
Sung ML, Fowden L (1968) trans-3-Hydroxy-l-proline: a constituent of Delonix regia. Phytochemistry 7:2061–2063
Szymanovicz G, Mercier O, Randoux A (1978) A new method of preparation and some properties of 3-hydroxyproline. Biochimie 60:499–503
Utagawa T (2004) Production of arginine by fermentation. J Nutr 134(10 Suppl):2854S–2857S
Wityak J, Gould SJ, Hein SJ, Keszler DA (1987) A 1,3-dipolar cycloaddition route to the 3(R)- and 3(S)-hydroxy-2(S)-arginines. J Org Chem 52:2179–2183
Yin X, Zabriskie TM (2004) VioC is a non-heme iron, α-ketoglutarate-dependent oxygenase that catalyzes the formation of 3S-hydroxy-l-arginine during viomycin biosynthesis. Chembiochem 5:1274–1277
Zheng X, Feng CG, Ye JL, Huang PQ (2005) Samarium diiodide promoted generation and asymmetric hydroxyalkylation of N, O-diprotected (3S)-3-pyrrolidinol 2-carbanions. Org Lett 7:553–556
Acknowledgements
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 15 K18677 (to R.H.).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 405 kb)
Rights and permissions
About this article
Cite this article
Hara, R., Kitatsuji, S., Yamagata, K. et al. Development of a multi-enzymatic cascade reaction for the synthesis of trans-3-hydroxy-l-proline from l-arginine. Appl Microbiol Biotechnol 100, 243–253 (2016). https://doi.org/10.1007/s00253-015-6992-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6992-4