Abstract
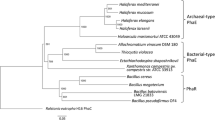
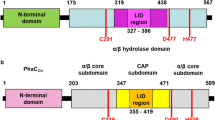
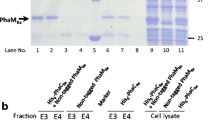
Polyhydroxyalkanoate (PHA) synthase from Bacillus cereus YB-4 (PhaRCYB4) catalyzes not only PHA polymerization but also alcoholytic cleavage of PHA chains. The alcoholysis activity of PhaRCYB4 is expressed when a hydroxyacyl-CoA monomer is absent but an alcohol compound is present. In this study, we performed alanine mutagenesis of the putative catalytic triad (Cys151, Asp306, and His335) in the PhaCYB4 subunit to identify the active site residues for polymerization and alcoholysis activities. Individual substitution of each triad residue with alanine resulted in loss of both polymerization and alcoholysis activities, suggesting that these residues are commonly shared between polymerization and alcoholysis reactions. The loss of activity was also observed following mutagenesis of the triad to other amino acids, except for one PhaRCYB4 mutant with a C151S substitution, which lost polymerization activity but still possessed cleavage activity towards PHA chains. The low-molecular-weight PHA isolated from the PhaRCYB4(C151S)-expressing strain showed a lower ratio of alcohol capping at the P(3HB) carboxy terminus than did that from the wild-type-expressing strain. This observation implies that hydrolysis activity of PhaRCYB4 might be elicited by the C151S mutation.






Similar content being viewed by others
References
Agus J, Kahar P, Hyakutake M, Tomizawa S, Abe H, Tsuge T, Satoh Y, Tajima K (2010) Unusual change in molecular weight of polyhydroxyalkanoate (PHA) during cultivation of PHA-accumulating Escherichia coli. Polym Degrad Stab 95:2250–2254
Arima J, Tanaka A, Morimoto M, Mori N (2014) Mutation of active site serine residue with cysteine displays change in acyl-acceptor preference of β-peptidyl aminopeptidase from Pseudomonas aeruginosa PAO1. Appl Microbiol Biotechnol 98:1631–1640
Ekici ÖD, Paetzel M, Dalbey RE (2008) Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration. Protein Sci 17:2023–2037
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hide WA, Chan L, Li WH (1992) Structure and evolution of the lipase superfamily. J Lipid Res 33:167–178
Hiroe A, Ushimaru K, Tsuge T (2013) Characterization of polyhydroxyalkanoate (PHA) synthase derived from Delftia acidovorans DS-17 and the influence of PHA production in Escherichia coli. J Biosci Bioeng 115:633–638
Hisano T, Kasuya K, Tezuka Y, Ishii N, Kobayashi T, Shiraki M, Oroudjevd E, Hansmad H, Iwata T, Doi Y, Saito T, Miki K (2006) The crystal structure of polyhydroxybutyrate depolymerase from Penicillium funiculosum provides insights into the recognition and degradation of biopolyesters. J Mol Biol 356:993–1004
Hyakutake M, Saito Y, Tomizawa S, Mizuno K, Tsuge T (2011) Polyhydroxyalkanoate (PHA) synthesis by class IV PHA synthases employing Ralstonia eutropha PHB−4 as host strain. Biosci Biotechnol Biochem 75:1615–1617
Hyakutake M, Tomizawa S, Mizuno K, Abe H, Tsuge T (2014) Alcoholytic cleavage of polyhydroxyalkanoate chains by class IV synthases induced by endogenous and exogenous ethanol. Appl Environ Microbiol 80:1421–1429
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Jia Y, Kappock TJ, Frick T, Sinskey AJ, Stubbe JA (2000) Lipases provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: characterization of the functional residues in Chromatium vinosum PHB synthase. Biochem 39:3927–3936
Jia Y, Yuan W, Wodzinska J, Park C, Sinskey AJ, Stubbe JA (2001) Mechanistic studies on class I polyhydroxybutyrate (PHB) synthase from Ralstonia eutropha: class I and III synthases share a similar catalytic mechanism. Biochem 40:1011–1019
Kato M, Bao HJ, Kang CK, Fukui T, Doi Y (1996) Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl Microbiol Biotechnol 45:363–370
Kawaguchi Y, Doi Y (1990) Structure of native poly(3-hydroxybutyrate) granules characterized by X-ray diffraction. FEMS Microbiol Lett 70:151–155
Kawaguchi Y, Doi Y (1992) Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromol 25:2324–2329
Kusaka S, Iwata T, Doi Y (1998) Microbial synthesis and physical properties of ultra-high-molecular-weight poly[(R)-3-Hydroxybutyrate]. Macromol Sci Part A 35:319–335
Matsusaki H, Abe H, Taguchi K, Fukui T, Doi Y (2000) Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl Microbiol Biotechnol 53:401–409
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
Mizuno K, Ohta A, Hyakutake M, Ichinomiya Y, Tsuge T (2010) Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym Degrad Stab 95:1335–1339
Rehm BH (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Slater SC, Voige WH, Dennis DE (1988) Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-β-hydroxybutyrate biosynthetic pathway. J Bacteriol 170:4431–4436
Stubbe J, Tian J (2003) Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat Prod Rep 20:445–457
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1053–1555
Tariq A, Hameed A, Bokhari H, Masood F (2014) Is atomic rearrangement of type IV PHA synthases responsible for increased PHA production? J Biomol Struc Dynam
Tomizawa S, Saito Y, Hyakutake M, Nakamura Y, Abe H, Tsuge T (2010) Chain transfer reaction catalyzed by various polyhydroxyalkanoate synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl Microbiol Biotechnol 87:1427–1435
Tomizawa S, Hyakutake M, Saito Y, Agus J, Mizuno K, Abe H, Tsuge T (2011) Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 12:2660–2666
Tomizawa S, Sato S, Lan JCW, Nakamura Y, Abe H, Tsuge T (2013) In vitro evidence of chain transfer to tetraethylene glycols in enzymatic polymerization of polyhydroxyalkanoate. Appl Microbiol Biotechnol 97:4821–4829
Tsuge T, Imazu S, Takase K, Taguchi S, Doi Y (2004) An extra large insertion in the polyhydroxyalkanoate synthase from Delftia acidovorans DS-17: its deletion effects and relation to cellular proteolysis. FEMS Microbiol Lett 231:77–83
Ushimaru K, Sangiambut S, Thomson N, Sivaniah E, Tsuge T (2013) New insights into activation and substrate recognition of polyhydroxyalkanoate synthase from Ralstonia eutropha. Appl Microbiol Biotechnol 97:1175–1182
Acknowledgments
We thank Dr. Y. Nakamura (Tokyo Institute of Technology) for NMR analysis. This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI 23310060) to T. Tsuge. M. Hyakutake was a recipient of a JSPS Young Scientist Fellowship (12J07940).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hyakutake, M., Tomizawa, S., Mizuno, K. et al. A common active site of polyhydroxyalkanoate synthase from Bacillus cereus YB-4 is involved in polymerization and alcoholysis reactions. Appl Microbiol Biotechnol 99, 4701–4711 (2015). https://doi.org/10.1007/s00253-014-6276-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6276-4