Abstract
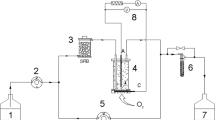
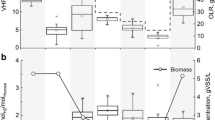
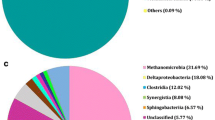
In this study, the impact of the hydrogen partial pressure on lactate degradation was investigated in a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1. To impose a change of the hydrogen partial pressure, formate was added to the reactor. Hydrogen results from the bioconversion of formate besides lactate in the liquid phase. In the presence of a hydrogen-consuming methanogen, this approach allows for a better estimation of low dissolved hydrogen concentrations than under conditions where hydrogen is supplied externally from the gas phase, resulting in a more accurate determination of kinetic parameters. A change of the hydrogen partial pressure from 1,200 to 250 ppm resulted in a threefold increase of the biomass-specific lactate consumption rate. The 50 % inhibition constant of hydrogen on lactate degradation was determined as 0.692 ± 0.064 μM dissolved hydrogen (831 ± 77 ppm hydrogen in the gas phase). Moreover, for the first time, the maximum biomass-specific lactate consumption rate of Desulfovibrio sp. G11 (0.083 ± 0.006 mol-Lac/mol-XG11/h) and the affinity constant for hydrogen uptake of Methanobrevibacter arboriphilus DH1 (0.601 ± 0.022 μM dissolved hydrogen) were determined. Contrary to the widely established view that the biomass-specific growth rate of a methanogenic coculture is determined by the hydrogen-utilizing partner; here, it was found that the hydrogen-producing bacterium determined the biomass-specific growth rate of the coculture grown on lactate and formate.








Similar content being viewed by others
References
Archer DB, Powell GE (1985) Dependence of the specific growth-rate of methanogenic mutualistic cocultures on the methanogen. Arch Microbiol 141(2):133–137. doi:10.1007/Bf00423273
Brill WJ, Wolin EA, Wolfe RS (1964) Anaerobic formate oxidation: a ferredoxin-dependent reaction. Science 144(3616):297–298. doi:10.1126/science.144.3616.297
Cussler EL (1997) Mass Transfer in Fluid Systems, 2nd edn. Cambridge University Press, New York
de Kok S, Meijer J, van Loosdrecht MCM, Kleerebezem R (2013) Impact of dissolved hydrogen partial pressure on mixed culture fermentations. Appl Microbiol Biot 97(6):2617–2625. doi:10.1007/s00253-012-4400-x
Dolfing J, Jiang B, Henstra AM, Stams AJ, Plugge CM (2008) Syntrophic growth on formate: a new microbial niche in anoxic environments. Appl Environ Microbiol 74(19):6126–6131. doi:10.1128/AEM. 01428-08
Fitz RM, Cypionka H (1991) Generation of a proton gradient in Desulfovibrio vulgaris. Arch Microbiol 155(5):444–448. doi:10.1007/bf00244959
Goodwin S, Giraldogomez E, Mobarry B, Switzenbaum MS (1991) Comparison of diffusion and reaction-rates in anaerobic microbial aggregates. Microbial Ecol 22(2):161–174. doi:10.1007/Bf02540221
Junicke H, Abbas B, Oentoro J, van Loosdrecht M, Kleerebezem R (2014) Absolute quantification of individual biomass concentrations in a methanogenic coculture. AMB Express 4:35. doi:10.1186/s13568-014-0035-x
Kleerebezem R, Stams AJM (2000) Kinetics of syntrophic cultures: a theoretical treatise on butyrate fermentation. Biotechnol Bioeng 67(5):529–543. doi:10.1002/(Sici)1097-0290(20000305)67:5<529::Aid-Bit4>3.0.Co;2-Q
Kleerebezem R, Van Loosdrecht MCM (2010) A generalized method for thermodynamic state analysis of environmental systems. Crit Rev Env Sci Tec 40(1):1–54. doi:10.1080/10643380802000974
Kristjansson JK, Schonheit P, Thauer RK (1982) Different Ks-values for hydrogen of methanogenic bacteria and sulfate reducing bacteria—an explanation for the apparent inhibition of methanogenesis by sulfate. Arch Microbiol 131(3):278–282. doi:10.1007/Bf00405893
Leadbetter JR, Breznak JA (1996) Physiological ecology of Methanobrevibacter cuticularis sp. nov. and Methanobrevibacter curvatus sp. nov., isolated from the hindgut of the termite Reticulitermes flavipes. Appl Environ Microbiol 62(10):3620–3631
Li X, McInerney MJ, Stahl DA, Krumholz LR (2011) Metabolism of H2 by Desulfovibrio alaskensis G20 during syntrophic growth on lactate. Microbiology 157(10):2912–2921. doi:10.1099/mic. 0.051284-0
McCarty PL, Bae J (2011) Model to couple anaerobic process kinetics with biological growth equilibrium thermodynamics. Environ Sci Technol 45(16):6838–6844. doi:10.1021/Es2009055
Meyer B, Kuehl J, Deutschbauer AM, Price MN, Arkin AP, Stahl DA (2013) Variation among Desulfovibrio species in electron transfer systems used for syntrophic growth. J Bacteriol 195(5):990–1004. doi:10.1128/JB.01959-12
Noguera DR, Brusseau GA, Rittmann BE, Stahl DA (1998) A unified model describing the role of hydrogen in the growth of Desulfovibrio vulgaris under different environmental conditions. Biotechnol Bioeng 59(6):732–746
Pankhania IP, Spormann AM, Hamilton WA, Thauer RK (1988) Lactate conversion to acetate, Co2 and H-2 in cell-suspensions of Desulfovibrio vulgaris (Marburg)—indications for the involvement of an energy driven reaction. Arch Microbiol 150(1):26–31. doi:10.1007/Bf00409713
Payot S, Guedon E, Cailliez C, Gelhaye E, Petitdemange H (1998) Metabolism of cellobiose by Clostridium cellulolyticum growing in continuous culture: evidence for decreased NADH reoxidation as a factor limiting growth. Microbiol-Uk 144:375–384
Pieulle L, Guigliarelli B, Asso M, Dole F, Bernadac A, Hatchikian EC (1995) Isolation and characterization of the pyruvate-ferredoxin oxidoreductase from the sulfate-reducing bacterium Desulfovibrio africanus. BBA-Protein Struct M 1250(1):49–59. doi:10.1016/0167-4838(95)00029-t
Plugge CM (2005) Anoxic media design, preparation, and considerations. Methods Enzymol 397:3–16. doi:10.1016/S0076-6879(05)97001-8
Robinson JA, Tiedje JM (1984) Competition between sulfate-reducing and methanogenic bacteria for H-2 under resting and growing conditions. Arch Microbiol 137(1):26–32. doi:10.1007/Bf00425803
Rodriguez J, Kleerebezem R, Lema JM, van Loosdrecht MCM (2006) Modeling product formation in anaerobic mixed culture fermentations. Biotechnol Bioeng 93(3):592–606. doi:10.1002/Bit.20765
Schauer NL, Brown DP, Ferry JG (1982) Kinetics of formate metabolism in Methanobacterium formicicum and Methanospirillum hungatei. Appl Environ Microbiol 44(3):549–554
Schink B (1997) Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol R 61(2):262
Sieber JR, McInerney MJ, Gunsalus RP (2012) Genomic insights into syntrophy: the paradigm for anaerobic metabolic cooperation. Annu Rev Microbiol 66:429–452. doi:10.1146/annurev-micro-090110-102844
Snoep JL, Joost M, Demattos T, Neijssel OM (1991) Effect of the energy-source on the NADH/NAD ratio and on pyruvate catabolism in anaerobic chemostat cultures of Enterococcus-faecalis NCTC-775. Fems Microbiol Lett 81(1):63–66
Traore AS, Fardeau ML, Hatchikian CE, Legall J, Belaich JP (1983) Energetics of growth of a defined mixed culture of Desulfovibrio vulgaris and Methanosarcina barkeri—interspecies hydrogen transfer in batch and continuous cultures. Appl Environ Microbiol 46(5):1152–1156
Uyeda K, Rabinowitz JC (1971) Pyruvate-ferredoxin oxidoreductase. 3. Purification and properties of the enzyme. J Biol Chem 246(10):3111–3119
Walker CB, He Z, Yang ZK, Ringbauer JA Jr, He Q, Zhou J, Voordouw G, Wall JD, Arkin AP, Hazen TC, Stolyar S, Stahl DA (2009) The electron transfer system of syntrophically grown Desulfovibrio vulgaris. J Bacteriol 191(18):5793–5801. doi:10.1128/JB.00356-09
Warikoo V, McInerney MJ, Robinson JA, Suflita JM (1996) Interspecies acetate transfer influences the extent of anaerobic benzoate degradation by syntrophic consortia. Appl Environ Microbiol 62(1):26–32
Weghoff MC, Bertsch J, Muller V (2014) A novel mode of lactate metabolism in strictly anaerobic bacteria. Environ Microbiol. doi:10.1111/1462-2920.12493
Willquist K, Nkemka VN, Svensson H, Pawar S, Ljunggren M, Karlsson H, Murto M, Hulteberg C, van Niel EWJ, Liden G (2012) Design of a novel biohythane process with high H2 and CH4 production rates. Int J Hydrogen Energy 37(23):17749–17762. doi:10.1016/j.ijhydene.2012.08.092
Zeikus JG, Henning DL (1975) Methanobacterium arbophilicum sp. nov—obligate anaerobe isolated from wetwood of living trees. A Van Leeuw J Microb 41(4):543–552. doi:10.1007/Bf02565096
Acknowledgments
The authors wish to thank Prof. Dr. AJM Stams and the Laboratory of Microbiology at Wageningen University for the supply of microbial cultures. The financial support of the Stichting voor de Technische Wetenschappen (STW, project number 11603) and Veolia Water is gratefully acknowledged.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junicke, H., Feldman, H., van Loosdrecht, M.C.M. et al. Impact of the hydrogen partial pressure on lactate degradation in a coculture of Desulfovibrio sp. G11 and Methanobrevibacter arboriphilus DH1. Appl Microbiol Biotechnol 99, 3599–3608 (2015). https://doi.org/10.1007/s00253-014-6241-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6241-2