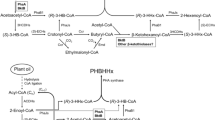
Abstract
The detection of glucoconjugated forms of monoterpene alcohols in rose petals in the late 1960s opened the new field of nonvolatile aroma precursors in flavor research. It is now well established that odorless glycosides represent a significant pool of aroma precursors in plants where they act as preformed but inactivated defense or attractive chemicals. Technical improvements in the separation and identification of plant secondary metabolites have provided a multitude of chemical structures, but functional characterization of glycosyltransferases that catalyze their formation lags behind. As technical efforts and costs for DNA sequencing dramatically dropped during the last decade, the number of plant genome sequences increased significantly, thus providing opportunities to functionally characterize the glycosyltransferase gene families in plants. These studies yielded the first glycosyltransferase genes that encode efficient biocatalysts for the production of monoterpene glucosides. They have applications in the food, feed, chemical, cosmetic, and pharmaceutical industries as slow release aroma chemicals.



Similar content being viewed by others
References
Allwood JW, Goodacre R (2010) An introduction to liquid chromatography-mass spectrometry instrumentation applied in plant metabolomic analyses. Phytochem Anal 21:33–47
Arakawa T, Yasuda H (1998) Oral deodorants for feces containing glycosides of fragrant compounds; From Jpn Kokai Tokkyo Koho, JP 10028724 A 19980203
Arend J, Warzecha H, Hefner T, Stöckigt J (2001) Utilizing genetically engineered bacteria to produce plant-specific glucosides. Biotechnol Bioeng 76:126–131
Asada K, Salim V, Masada-Atsumi S, Edmunds E, Nagatoshi M, Terasaka K, Mizukami H, De Luca V (2013) A 7-deoxyloganetic acid glucosyltransferase contributes a key step in secologanin biosynthesis in Madagascar periwinkle. Plant Cell 25:4123–4134
Bachhawat P, Mishra S, Bhatia Y, Bisaria VS (2004) Enzymatic synthesis of oligosaccharides alkyl and terpenyl glucosides by recombinant Escherichia coli-expressed Pichia etchellsii beta-glucosidase II. Appl Biochem Biotechnol 118:269–282
Bai X, Mou D, Dong W, Peng G, Gong R, Wang L, Zhang Y (2012) Method for compensating flavor of low tar cigarettes; From Faming Zhuanli Shenqing, CN 102423114 A 20120425
Behrens M, Brockhoff A, Batram C, Kuhn C, Appendino G, Meyerhof W (2009) The human bitter taste receptor hTAS2R50 is activated by the two natural bitter terpenoids andrographolide and amarogentin. J Agric Food Chem 57:9860–9866
Berger RG (2009) Biotechnology of flavours–the next generation. Biotechnol Lett 31:1651–1659
Berkov S, Mutafova B, Christen P (2014) Molecular biodiversity and recent analytical developments: A marriage of convenience. Biotechnol Adv 32:1102–1110
Biere A, Marak HB, van Damme JM (2004) Plant chemical defense against herbivores and pathogens: generalized defense or trade-offs? Oecologia 140:430–441
Bohlmann J, Keeling C (2008) Terpenoid biomaterials. Plant J 54:656–669
Bönisch F, Frotscher J, Stanitzek S, Rühl E, Wüst M, Bitz O, Schwab W (2014a) Activity-based profiling of a physiologic aglycone library reveals sugar acceptor promiscuity of family 1 UDP-glucosyltransferases from grape. Plant Physiol 166:23–39
Bönisch F, Frotscher J, Stanitzek S, Rühl E, Wüst M, Bitz O, Schwab W (2014b) A UDP-glucose:monoterpenolglucosyltransferase adds to the chemical diversity of the grapevine metabolome. Plant Physiol 165:561–581
Bouvier F, Rahier A, Camara B (2005) Biogenesis molecular regulation and function of plant isoprenoids. Prog Lipid Res 44:357–429
Bowles D (2002) A multigene family of glycosyltransferases in a model plant Arabidopsis thaliana. Biochem Soc Trans 30:301–306
Bowles D, Lim EK, Poppenberger B, Vaistij FE (2006) Glycosyltransferases of lipophilic small molecules. Annu Rev Plant Biol 57:567–597
Bülter T, Elling L (1999) Enzymic synthesis of nucleotide sugars. Glycoconj J 16:147–159
Burse A, Frick S, Discher S, Tolzin-Banasch K, Kirsch R, Strauss A, Kunert M, Boland W (2009) Always being well prepared for defense: the production of deterrents by juvenile Chrysomelina beetles (Chrysomelidae). Phytochemistry 70:1899–1909
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B (2009) The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Res 37:D233–8
Caputi L, Aprea E (2011) Use of terpenoids as natural flavouring compounds in food industry. Recent Pat Food Nutr Agric 3:9–16
Caputi L, Lim EK, Bowles DJ (2008) Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Eur J Chem 14:656–6662
Clark GS (1998) Geraniol. Perfum Flavor 23:19–25
Coutinho PM, Deleury E, Davies GJ, Henrissat B (2003) An evolving hierarchical family classification for glycosyltransferases. J Mol Biol 328:307–317
De Roode BM, Oliehoek L, van der Padt A, Franssen MC, Boom RM (2001) Downstream processing of enzymatically produced geranyl glucoside. Biotechnol Prog 17:881–886
Desmet T, Soetaert W, Bojarová P, Křen V, Dijkhuizen L, Eastwick-Field V, Schiller A (2012) Enzymatic glycosylation of small molecules: challenging substrates require tailored catalysts. Eur J Chem 18:10786–10801
Dong T, Hwang I (2014) Contribution of ABA UDP-glucosyltransferases in coordination of ABA biosynthesis and catabolism for ABA homeostasis. Plant Signal Behav 29(9 pii):e28888
Donho M, Kimura T (1996) Perfume precursor-containing compositions for massage; From Jpn Kokai Tokkyo Koho, JP 08188525 A 19960723
Donho M, Kimura T, Sekya M (1996) Bath preparations containing perfume precursors and the precursor-hydrolyzing enzymes; From Jpn Kokai Tokkyo Koho, JP 08188529 A 19960723
Duncan SH, Leitch EC, Stanley KN, Richardson AJ, Laven RA (2004) Effects of esculin and esculetin on the survival of Escherichia coli O157 in human faecal slurries continuous-flow simulations of the rumen and colon and in calves. Br J Nutr 91:749–755
Dinda B, Debnath S, Banik R (2011) Naturally occurring iridoids and secoiridoids. An updated review, part 4. Chem Pharm Bull 49:803-833
Dunkel A, Steinhaus M, Kotthoff M, Nowak B, Krautwurst D, Schieberle P, Hofmann T (2014) Nature’s chemical signatures in human olfaction: a foodborne perspective for future biotechnology. Angew Chem Int Ed Engl 53:7124–7143
Feng T, Xiao Z, Tian H (2009) Recent patents in flavor microencapsulation. Recent Pat Food Nutr Agric 1:193–202
Francis MJ, Allcock C (1969) Monoterpene beta-D-glucosides in roses: isolation and formation from DL-2-14C mevalonate. Biochem J 113:38
Gachon CM, Langlois-Meurinne M, Saindrenan P (2005) Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci 10:542–549
Genus JMC (2003) Stevioside. Phytochemistry 64:913–921
Gershenzon J, Dudareva N (2007) The function of terpene natural products in the natural world. Nat Chem Biol 3:408–414
Geu-Flores F, Sherden NH, Courdavault V, Burlat V, Glenn WS, Wu C, Nims E, Cui Y, O’Connor SE (2012) An alternative route to cyclic terpenes by reductive cyclization in iridoid biosynthesis. Nature 492:138–142
Gray MJ, Chang D, Zhang Y, Liu J, Bensoussan A (2010) Development of liquid chromatography/mass spectrometry methods for the quantitative analysis of herbal medicine in biological fluids: a review. Biomed Chromatogr 24:91–103
Grice EA, Segre JA (2011) The skin microbiome. Nat Rev Microbiol 9:244–253
Hansen KS, Kristensen C, Tattersall DB, Jones PR, Olsen CE, Bak S, Møller BL (2003) The in vitro substrate regiospecificity of recombinant UGT85B1 the cyanohydrin glucosyltransferase from Sorghum bicolor. Phytochemistry 64:143–151
He XZ, Wang X, Dixon RA (2006) Mutational analysis of the Medicago glycosyltransferase UGT71G1 reveals residues that control regioselectivity for (iso)flavonoid glycosylation. J Biol Chem 281:34441–34447
Hefner T, Arend J, Warzecha H, Siems K, Stöckigt J (2002) Arbutin synthase a novel member of the NRD1ß glycosyltransferase family is a unique multifunctional enzyme converting various natural products and xenobiotics. Bioorg Med Chem 10:1731–4171
Herrmann A (2007) Controlled release of volatile compounds under mild reaction conditions: from nature to consumer products. Angew Chem 119:5938–5967
Höfer R, Dong L, André F, Ginglinger JF, Lugan R, Gavira C, Grec S, Lang G, Memelink J, Van der Krol S, Bouwmeester H, Werck-Reichhart D (2013) Geraniol hydroxylase and hydroxygeraniol oxidase activities of the CYP76 family of cytochrome P450 enzymes and potential for engineering the early steps of the (seco)iridoid pathway. Metab Eng 20:221–232
Ikemoto T, Okabe B-I, Mimura K, Kitahara T (2002) Formation of fragrant materials from odourless glycosidically-bound volatiles on skin microflora (Part 1). Flavour Fragr J 17:452–455
Ikemoto T, Mimura K, Kitahara T (2003) Formation of fragrant materials from odourless glycosidically-bound volatiles on skin microflora (Part 2). Flavour Fragr J 18:45–47
Itkin M, Heinig U, Tzfadia O, Bhide AJ, Shinde B, Cardenas PD, Bocobza SE, Unger T, Malitsky S, Finkers R, Tikunov Y, Bovy A, Chikate Y, Singh P, Rogachev I, Beekwilder J, Giri AP, Aharoni A (2013) Biosynthesis of antinutritional alkaloids in Solanaceous crops is mediated by clustered genes. Science 341:175–179
Iwamoto M, Gocho S, Fujita A, Waku M, Pponda T (1994) Fragrance enhancers for plants; From Jpn Kokai Tokkyo Koho, JP 06336401 A 19941206
Jánváry L, Hoffmann T, Pfeiffer J, Hausmann L, Töpfer R, Fischer TC, Schwab W (2009) A double mutation in the anthocyanin 5-O-glucosyltransferase gene disrupts enzymatic activity in Vitis vinifera L. J Agric Food Chem 57:3512–3518
Jelassi A, Zardi-Bergaoui A, Ben Nejma A, Belaiba M, Bouajila J, Ben Jannet H (2014) Two new unusual monoterpene acid glycosides from Acacia cyclops with potential cytotoxic activity. Bioorg Med Chem Lett 24:3777–3781
Jones PR, Moller BL, Hoj PB (1999) The UDP-glucose:p-hydroxymandelonitrile-O-glucosyltransferase that catalyzes the last step in synthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor Isolation cloning heterologous expression and substrate specificity. J Biol Chem 274:35483–35491
Kimura T, Hattori N (1998) Enhancement of plant fragrance enhancers containing ß-D-galactosides and the plants with enhanced fragrance; From Jpn Kokai Tokkyo Koho, JP 10060472 A 19980303
Kimura T, Ito H, Sekiya M, Donho M, Nakajima H (1997a) Odor-absorbing garments; From Jpn Kokai Tokkyo Koho, JP 09158041 A 19970617
Kimura T, Ito H, Sekiya M, Donho M, Nakajima H (1997b) Deodorants containing perfume precursors; From Jpn Kokai Tokkyo Koho, JP 09154930 A 19970617
Kimura T, Ito H, Sekiya M, Donho M, Nakajima H (1997c) Beddings containing perfume precursors to mask perspiration-related odor; From Jpn Kokai Tokkyo Koho, JP 09154677 A 19970617
Kubo A, Arai Y, Nagashima S, Yoshikawa T (2004) Alteration of sugar donor specificities of plant glycosyltransferases by a single point mutation. Arch Biochem Biophys 429:198–203
Lairson LL, Henrissat B, Davies GJ, Withers SG (2008) Glycosyltransferases: structures functions and mechanisms. Annu Rev Biochem 77:521–555
Lao J, Oikawa A, Bromley JR, McInerney P, Suttangkakul A, Smith-Moritz AM, Plahar H, Chiu TY, González Fernández-Niño SM, Ebert B, Yang F, Christiansen KM, Hansen SF, Stonebloom S, Adams PD, Ronald PC, Hillson NJ, Hadi MZ, Vega-Sánchez ME, Loqué D, Scheller HV, Heazlewood JL (2014) The plant glycosyltransferase clone collection for functional genomics. Plant J 79:517–529
Lim EK, Baldauf S, Li Y, Elias L, Worrall D, Spencer SP, Jackson RG, Taguchi G, Ross J, Bowles DJ (2003) Evolution of substrate recognition across a multigene family of glycosyltransferases in Arabidopsis. Glycobiology 13:139–145
Lim EK, Ashford DA, Hou B, Jackson RG, Bowles DJ (2004) Arabidopsis glycosyltransferases as biocatalysts in fermentation for regioselective synthesis of diverse quercetin glucosides. Biotech Bioeng 87:623–631
Lombard V, GolacondaRamulu H, Drula E, Coutinho PM, Henrissat B (2014) The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res 42:D490–5
Marks LE, Veldhuizen MG, Shepard TG, Shavit AY (2012) Detecting gustatory-olfactory flavor mixtures: models of probability summation. Chem Senses 37:263–277
Masada S, Kawase Y, Nagatoshi M, Oguchi Y, Terasaka K, Mizukami H (2007) An efficient chemoenzymatic production of small molecule glucosides with in situ UDP-glucose recycling. FEBS Lett 581:2562–2566
Mateo JJ, Jiménez M (2000) Monoterpenes in grape juice and wines. J Chromatogr A 881:557–567
Mercer DK, Robertson J, Wright K, Miller L, Smith S, Stewart CSO, Neil DA (2013) A prodrug approach to the use of coumarins as potential therapeutics for superficial mycoses. PLoS One 8(11):e80760
Michael TP, Jackson S (2013) The First 50 Plant Genomes. Plant Genome 6:2
Nagashima S, Tomo S, Orihara Y, Yoshikawa T (2004) Cloning and characterization of glucosyltransferase cDNA from Eucalyptus perriniana cultured cells. Plant Biotechnol 21:343–348
Nagatoshi M, Terasaka K, Nagatsu A, Mizukami H (2011) Iridoid-specific glucosyltransferase from Gardenia jasminoides. J Biol Chem 286:32866–32874
Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ (2006) Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J 25:1396–1405
Ostrowski M, Jakubowska A (2014) UDP-glycosyltransferases of plant hormones. Adv Cell Biol 2:271–288
Rather MY, Mishra S (2013) ß-Glycosidases: An alternative enzyme based method for synthesis of alkyl-glycosides. Sustainable Chemical Processes 1:7
Rivas F, Parra A, Martinez A, Garcia-Granados A (2013) Enzymatic glycosylation of terpenoids. Phytochem Rev 12:327–339
Rocha SM, Coutinho P, Delgadillo I, Dias Cardoso A, Coimbra MA (2004) Effect of enzymatic aroma release on the volatile compounds of white wines presenting different aroma potentials. J Sci Food Agric 85:199–205
Rowan DD (2011) Volatile Metabolites. Metabolites 1:41–63
Schmidt E, Wanner J, Hiiferl M, Jirovetz L, Buchbauer G, Gochev V, Girova T, Stoyanova A, Geissler M (2012) Chemical composition olfactory analysis and antibacterial activity of Thymus vulgaris chemotypes geraniol, 4-thujanol/terpinen-4-ol, thymol and linalool cultivated in southern France. Nat Prod Commun 7:1095–1098
Schwab W, Davidovich-Rikanati R, Lewinsohn E (2008) Biosynthesis of plant-derived flavor compounds. Plant J 54:712–732
Serra S, Fuganti C, Brenna E (2005) Biocatalytic preparation of natural flavours and fragrances. Trends Biotechnol 23:193–198
Singh S, Phillips GN Jr, Thorson JS (2012) The structural biology of enzymes involved in natural product glycosylation. Nat Prod Rep 29:1201–1237
Song MC, Kim E, Ban YH, Yoo YJ, Kim EJ, Park SR, Pandey RP, Sohng JK, Yoon YJ (2013) Achievements and impacts of glycosylation reactions involved in natural product biosynthesis in prokaryotes. Appl Microbiol Biotechnol 97:5691–5704
Stahl Biskup E, Intert F, Holthuijzen J, Stengele M, Schulz G (1993) Glycosidically bound volatiles – a review 1986–91. Flav Frag J 8:61–80
Tanaka M, Kotsuna K (1993) Fragrant compositions containing a single fragrant substance; From Jpn Kokai Tokkyo Koho , JP 05239491 A 19930917
Thibodeaux CJ, Melançon CE, Liu HW (2007) Unusual sugar biosynthesis and natural product glycodiversification. Nature 446:1008–1016
Tikunov YM, Molthoff J, de Vos RCH, Beekwilder J, van Houwelingen A, van der Hoooft JJJ, Vries MN, Labrie CW, Verkerke W, van de Gees H, Zamora MV, Presa S, Rambia JL, Granell A, Hall RD, Bovy AG (2013) NON-SMOKY GLYCOSYLTRANSFERASE1 prevents the release of smoky aroma from tomato fruit. Plant Cell 25:3067–3078
Toshima K, Tatsuta K (1993) Recent progress in O-glycosylation methods and its application to natural products synthesis. Chem Rev 93:1503–1531
Tundis R, Loizzo MR, Menichini F, Statti GA, Menichini F (2008) Biological and pharmacological activities of iridoids: recent developments. Mini Rev Med Chem 8:399–420
Unligil UM, Rini JM (2000) Glycosyltransferase structure and mechanism. Curr Opin Struct Biol 10:510–517
Vera Saltos MB, Naranjo Puente BF, Malafronte N, Braca A (2014) A new monoterpene glycoside from Siparuna thecaphora. Nat Prod Res 28:57–60
Vilanova M, Genisheva Z, Bescansa L, Masa A, Oliveira JM (2012) Changes in free and bound fractions of aroma compounds of four Vitis vinifera cultivars at the last ripening stages. Phytochemistry 74:196–205
Wang X (2009) Structure mechanism and engineering of plant natural product glycosyltransferases. FEBS Lett 583:3303–3309
Wang D, Yoshimura T, Kubota K, Kobayashi A (2000) Analysis of glycosidically bound aroma precursors in tea leaves 1. Qualitative and quantitative analyses of glycosides with aglycons as aroma compounds. J Agric Food Chem 48:5411–5418
Wang Q, Grkovic T, Font J, Bonham S, Pouwer RH, Bailey CG, Moran AM, Ryan RM, Rasko JE, Jormakka M, Quinn RJ, Holst J (2014) Monoterpene glycoside ESK246 from Pittosporum targets LAT3 amino acid transport and prostate cancer cell growth. ACS Chem Biol 9:1369–1376
Wen YQ, He F, Zhu BQ, Lan YB, Pan QH, Li CY, Reeves MJ, Wang J (2014) Free and glycosidically bound aroma compounds in cherry (Prunus avium L). Food Chem 152:29–36
Williams PJ, Strauss CR, Wilson B, Massy-Westropp RA (1982) Studies on the hydrolysis of Vitis vinifera monoterpene precursor compounds and model monoterpene ß-D-glucosides rationalizing the monoterpene composition of grapes. J Agric Food Chem 30:1219–1223
Winterhalter P, Skouroumounis GK (1997) Glycoconjugated aroma compounds: occurrence role and biotechnological transformation. Adv Biochem Eng Biotechnol 55:73–105
Xiao J, Muzashvili TS, Georgiev MI (2014) Advances in the biotechnological glycosylation of valuable flavonoids. Biotechnol Adv 32:1145–1156
Yamada T, Jinsawa M, Sakata K, Usui T, Watanabe S, Yamagishi M, Ooishi K (1998) Unpleasant odor-inhibiting compositions containing fragrance inclusion compounds and sanitary and pet-care products using them; From Jpn Kokai Tokkyo Koho, JP 10263062 A 19981006
Yang T, Stoopen G, Yalpani N, Vervoort J, de Vos R, Voster A, Verstappen FW, Bouwmeester HJ, Jongsma MA (2011) Metabolic engineering of geranic acid in maize to achieve fungal resistance is compromised by novel glycosylation patterns. Metab Eng 13:414–425
Yang YH, Huang SZ, Han YL, Yuan HY, Gu CS, Zhao YH (2014) Base substitution mutations in uridinediphosphate-dependent glycosyltransferase 76G1 gene of Stevia rebaudiana causes the low levels of rebaudioside A: mutations in UGT76G1, a key gene of steviol glycosides synthesis. Plant Physiol Biochem 80:220–225
Yauk YK, Ged C, Wang MY, Matich AJ, Tessarotto L, Cooney JM, Chervin C, Atkinson RG (2014) Manipulation of flavour and aroma compound sequestration and release using a glycosyltransferase with specificity for terpene alcohols. Plant J doi: 10.1111/tpj12634 [Epub ahead of print]
Yonekura-Sakakibara K (2009) Functional genomics of family 1 glycosyltransferases in Arabidopsis. Plant Biotechnol 26:267–274
Yonekura-Sakakibara K, Hanada K (2011) An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J 66:182–193
Zhu X, Fang ZH (2014) New monoterpene glycosides from the root cortex of Paeonia suffruticosa and their potential anti-inflammatory activity. Nat Prod Res 28:301–305
Acknowledgments
We are grateful for the financial support by Deutsche Forschungsgemeinschaft (DFG) SCHW634/17. APG acknowledges the Alexander von Humbolt Foundation (Bonn, Germany) for a research fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schwab, W., Fischer, T.C., Giri, A. et al. Potential applications of glucosyltransferases in terpene glucoside production: impacts on the use of aroma and fragrance. Appl Microbiol Biotechnol 99, 165–174 (2015). https://doi.org/10.1007/s00253-014-6229-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6229-y