Abstract
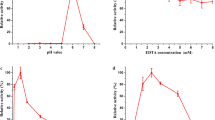
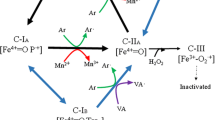
The manganese peroxidase gene family (mnps) is a part of the ligninolytic system of Pleurotus ostreatus. This gene family is comprised of nine members, mnp1–9, encoding short manganese peroxidases (short-MnPs) or versatile peroxidases (VPs). We show that unlike in Mn2+-amended glucose–peptone (GP) medium, where redundancy among mnps was reported, in Mn2+-deficient GP medium mnp4 [encoding versatile peroxidase isoenzyme 4 (VP4)] has a key and nonredundant function. The abundance of mnps transcripts at time points corresponding to the tropophase (active growth), early idiophase, and idiophase indicates that mnp4 is the predominantly expressed mnp gene and that its relative predominance is dependent on the age of the culture. In this medium, azo dye, Orange II (OII) decolorization occurs only during the idiophase and a Δmnp4 strain showed a drastic reduction in this decolorization. Three degradation metabolites were identified by liquid chromatography-mass spectroscopy (LC-MS), indicating both asymmetric and symmetric enzymatic cleavage of the azo-bond. In addition, the culture filtrate of Δmnp4 showed negligible values of oxidation capability of four typical VP substrates: Mn2+, 2,6-dimethoxyphenol, phenol red, and Reactive Black 5 (RB5), compared to the wild-type strain PC9. We concluded that under Mn2+-deficient GP culture, VP4 (encoded by mnp4) is the main active ligninolytic enzyme able to oxidize Mn2+ as well as high and low redox potential aromatic substrate, including dyes. Furthermore, other VPs/MnPs do not compensate for the lack of VP4 activity.






Similar content being viewed by others
References
Buswell JA, Odier E (1987) Lignin biodegradation. Crit Rev Biotechnol 6:1–60
Cohen R, Persky L, Hadar Y (2002) Biotechnological applications and potential of wood-degrading mushrooms of the genus Pleurotus. Appl Microbiol Biotechnol 58:582–594
Daâssi D, Frikha F, Zouari-Mechichi H, Belbahri L, Woodward S, Mechichi T (2012) Application of response surface methodology to optimize decolourization of dyes by the laccase-mediator system. J Environ Manag 108:84–91
Doyle WA, Blodig W, Veitch NC, Piontek K, Smith AT (1998) Two substrate interaction sites in lignin peroxidase revealed by site-directed mutagenesis. Biochemistry 37:15097–15105
Fernández-Fueyo E, Castanera ER, Ruiz-Dueñas FJ, López-Lucendo MF, Ramírez A, Pisabarro AG, Martínez AT (2014a) Ligninolytic peroxidase gene expression by Pleurotus ostreatus: differential regulation in lignocellulose medium and effect of temperature and pH. Fungal Genet Biol. doi:10.1016/j.fgb.2014.02.003
Fernández-Fueyo E, Ruiz-Dueñas FJ, Martínez MJ, Romero A, Hammel KE, Medrano FJ, Martínez AT (2014b) Ligninolytic peroxidase genes in the oyster mushroom genome: heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol Biofuels 7:2–23
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo A, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, John FS, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science 336:1715–1719
García-Moñtano J, Domènech X, García-Hortal JA, Torrades F, Peral J (2008) The testing of several biological and chemical coupled treatments for cibacron red FN-R azo dye removal. J Hazard Mater 154:484–490
Giardina P, Palmieri G, Fontanella B, Rivieccio V, Sannia G (2000) Manganese peroxidase isoenzymes produced by Pleurotus ostreatus grown on wood sawdust. Arch Biochem Biophys 376:171–179
Glenn JK, Gold MH (1983) Decolorization of several polymeric dyes by the lignin-degrading basidiomycete Phanerochaete chrysosporium. Appl Environ Microbiol 45:1741–1747
Gold MH, Youngs HL, Gelpke MDS (2000) Manganese peroxidase. Met Ions Biol Syst 37:559–586
Hammel KE, Cullen D (2008) Role of fungal peroxidases in biological ligninolysis. Curr Opin Plant Biol 11:349–355
Hildén K, Martínez AT, Hatakka A, Lundell T (2005) The two manganese peroxidases Pr-MnP2 and Pr-MnP3 of Phlebia radiata, a lignin-degrading basidiomycete, are phylogenetically and structurally divergent. Fungal Genet Biol 42:403–419
Hofrichter M, Ullrich R, Pecyna MJ, Liers C, Lundell T (2010) New and classic families of secreted fungal heme peroxidases. Appl Microbiol Biotechnol 87:871–897
Kamitsuji H, Honda Y, Watanabe T, Kuwahara M (2004) Production and induction of manganese peroxidase isozymes in a white-rot fungus Pleurotus ostreatus. Appl Microbiol Biotechnol 65:287–294
Kamitsuji H, Watanabe T, Honda Y, Kuwahara M (2005) Direct oxidation of polymeric substrates by multifunctional manganese peroxidase isoenzyme from Pleurotus ostreatus without redox mediators. Biochem J 386:387–393
Liang X, Zhong Y, Zhu S, Zhu J, Yuan P, He H, Zhang J (2010) The decolorization of acid Orange II in non-homogeneous Fenton reaction catalyzed by natural vanadium–titanium magnetite. J Hazard Mater 181:112–120
López C, Moreira MT, Feijoo G, Lema JM (2004a) Dye decolorization by manganese peroxidase in an enzymatic membrane bioreactor. Biotechnol Prog 20:74–81
López C, Valade AG, Combourieu B, Mielgo M, Bouchon B, Lema JM (2004b) Mechanism of enzymatic degradation of the azo dye Orange II determined by ex situ H-1 nuclear magnetic resonance and electrospray ionization-ion trap mass spectrometry. Anal Biochem 335:135–149
Lundell TK, Mäkelä MR, Hildén K (2010) Lignin-modifying enzymes in filamentous basidiomycetes—ecological, functional and phylogenetic review. J Basic Microbiol 50:5–20
Martínez AT (2002) Molecular biology and structure–function of lignin-degrading heme peroxidases. Enzym Microb Technol 30:425–444
Martínez MJ, Ruiz-Dueñas FJ, Guillén F, Martínez AT (1996) Purification and catalytic properties of two manganese peroxidase isoenzymes from Pleurotus eryngii. Eur J Biochem 237:424–432
Mielgo I, López C, Moreira MT, Feijoo G, Lema JM (2003) Oxidative degradation of azo dyes by manganese peroxidase under optimized conditions. Biotechnol Prog 19:325–331
Morales M, Mate MJ, Romero A, Martínez MJ, Martínez AT, Ruiz-Dueñas FJ (2012) Two oxidation sites for low redox potential substrates: a direct mutagenesis, kinetic, and, crystallographic study on Pleurotus eryngii versatile peroxidase. J Biol Chem 287:41053–41067
Morgenstern I, Klopman S, Hibbett DS (2008) Molecular evolution and diversity of lignin degrading heme peroxidases in the Agaricomycetes. J Mol Evol 66:243–257
Pan H, Feng J, Cerniglia CE, Chen H (2011) Effects of Orange II and Sudan III azo dyes and their metabolites on Staphylococcus aureus. J Ind Microbiol Biotechnol 38:1729–1738
Pérez-Boada M, Ruiz-Dueñas FJ, Pogni R, Basosi R, Choinowski T, Martínez MJ, Piontek K, Martínez AT (2005) Versatile peroxidase oxidation of high redox potential aromatic compounds: site-directed mutagenesis, spectroscopic and crystallographic investigation of three long-range electron transfer pathways. J Mol Biol 354:385–402
Pezzella C, Lettera V, Piscitelli A, Giardina P, Sannia G (2013) Transcriptional analysis of Pleurotus ostreatus laccase genes. Appl Microbiol Biotechnol 97:705–717
Ruiz-Dueñas FJ, Morales M, Pérez-Boada M, Choinowski T, Martínez MJ, Piontek K, Martínez AT (2007) Manganese oxidation site in Pleurotus eryngii versatile peroxidase: a site-directed mutagenesis, kinetic, and crystallographic study. Biochemistry 46:66–77
Ruiz-Dueñas FJ, Morales M, Mate MJ, Romero A, Martínez MJ, Smith AT, Martínez AT (2008) Site-directed mutagenesis of the catalytic tryptophan environment in Pleurotus eryngii versatile peroxidase. Biochemistry 47:1685–1695
Ruiz-Dueñas FJ, Fernández E, Martínez MJ, Martínez AT (2011) Pleurotus ostreatus heme peroxidases: an in silico analysis from the genome sequence to the enzyme molecular structure. C R Biol 334:795–805
Salame TM, Yarden O, Hadar Y (2010) Pleurotus ostreatus manganese-dependent peroxidase silencing impairs decolourization of Orange II. Microb Biotechnol 3:93–106
Salame TM, Knop D, Tal D, Levinson D, Yarden O, Hadar Y (2012) Predominance of a versatile-peroxidase-encoding gene, mnp4, as demonstrated by gene replacement via a gene targeting system for Pleurotus ostreatus. Appl Environ Microbiol 78:5341–5352
Salame TM, Knop D, Levinson D, Mabjeesh SJ, Yarden O, Hadar Y (2013) Redundancy among manganese-peroxidases in Pleurotus ostreatus. Appl Environ Microbiol 79:2405–2415
Salame TM, Knop D, Levinson D, Mabjeesh SJ, Yarden O, Hadar Y (2014) Inactivation of a Pleurotus ostreatus versatile peroxidase-encoding gene (mnp2) results in reduced lignin degradation. Environ Microbiol 16:265–277
Sanchez C (2010) Cultivation of Pleurotus ostreatus and other edible mushrooms. Appl Microbiol Biotechnol 85:1321–1337
Saparrat M, Balatti PA, Martínez MJ, Jurado M (2010) Differential regulation of laccase gene expression in Coriolopsis. Fungal Biol 114:999–1006
Sarkar S, Martínez AT, Martínez MJ (1997) Biochemical and molecular characterization of a manganese peroxidase isoenzyme from Pleurotus ostreatus. BBA-Protein Struct Mol 1339:23–30
Smith AT, Veitch NC (1998) Substrate binding and catalysis in heme peroxidases. Curr Opin Chem Biol 2:269–278
Solís M, Solís A, Pérez HI, Manjarrez N, Flores M (2012) Microbial decolouration of azo dyes: a review. Process Biochem 47:1723–1748
Stajić M, Vukojević J, Duletić-Laušević S (2009) Biology of Pleurotus eryngii and role in biotechnological processes: a review. Crit Rev Biotechnol 29:55–66
Tsukihara T, Honda Y, Sakai R, Watanabe T, Watanabe T (2008) Mechanism for oxidation of high-molecular-weight substrates by a fungal versatile peroxidase, MnP2. Appl Environ Microbiol 74:2873–2881
Urra J, Sepúlveda L, Contreras E, Palma C (2006) Screening of static culture and comparison of batch and continuous culture for the textile dye biological decolorization by Phanerochaete chrysosporium. Braz J Chem Eng 23:281–290
Yakovlev IA, Hietala AM, Courty P-E, Lundell T, Solheim H, Fossdal CG (2013) Genes associated with lignin degradation in the polyphagous white-rot pathogen Heterobasidion irregulare show substrate-specific regulation. Fungal Genet Biol 56:17–24
Zhang A, Fang L, Wang J, Liu W (2013) Enzymatic decolorization of Orange II: optimization by response surface methodology and pathway. Environ Prog Sustain Energy 32:294–301
Zhao X, Lu Y, Hardin I (2005) Determination of biodegradation products from sulfonated dyes by Pleurotus ostreatus using capillary electrophoresis coupled with mass spectrometry. Biotechnol Lett 27:69–72
Zhao X, Lu Y, Phillips DR, Hwang H-M, Hardin IR (2007) Study of biodegradation products from azo-dyes in fungal degradation by capillary electrophoresis/electrospray mass spectrometry. J Chromatogr A 1159:217–224
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 183 kb)
Rights and permissions
About this article
Cite this article
Knop, D., Ben-Ari, J., Salame, T.M. et al. Mn2+-deficiency reveals a key role for the Pleurotus ostreatus versatile peroxidase (VP4) in oxidation of aromatic compounds. Appl Microbiol Biotechnol 98, 6795–6804 (2014). https://doi.org/10.1007/s00253-014-5689-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5689-4