Abstract
Organoids are a novel three-dimensional stem cells’ culture system that allows the in vitro recapitulation of organs/tissues structure complexity. Pluripotent and adult stem cells are included in a peculiar microenvironment consisting of a supporting structure (an extracellular matrix (ECM)-like component) and a cocktail of soluble bioactive molecules that, together, mimic the stem cell niche organization. It is noteworthy that the balance of all microenvironmental components is the most critical step for obtaining the successful development of an accurate organoid instead of an organoid with heterogeneous morphology, size, and cellular composition. Within this system, mechanical forces exerted on stem cells are collected by cellular proteins and transduced via mechanosensing—mechanotransduction mechanisms in biochemical signaling that dictate the stem cell specification process toward the formation of organoids. This review discusses the role of the environment in organoids formation and focuses on the effect of physical components on the developmental system. The work starts with a biological description of organoids and continues with the relevance of physical forces in the organoid environment formation. In this context, the methods used to generate organoids and some relevant published reports are discussed as examples showing the key role of mechanosensing–mechanotransduction mechanisms in stem cell-derived organoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Organoids
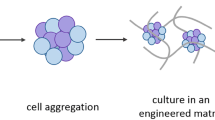
In recent decades, there has been significant advancement of three-dimensional (3D)-cell culture systems to address the limitations of two-dimensional (2D) culture systems and to better mimic tissue structure and functionality. It is now commonly recognized that cells grown in 3D environments develop more specific biological multicellular structures than cells in 2D cultures, which typically acquire a monolayer morphology (Argentati et al. 2020a). In this context, stem cells, due to the staminal properties of self-renewal and differentiation toward cell types from multiple lineages, have been considered as useful tool for the building of faithful 3D models. When cultured in an appropriate environment, stem cells accomplish their intrinsic developmental programs, which result in self-organization and generation of biologically relevant 3D structures that recapitulate in vitro several features of tissues and organs and are therefore called “organoids” (Brassard and Lutolf 2019) (Figs. 1, 2).
Schematic of organoids developmental process. a Pluripotent stem cells (PSCs) require a first step of induction toward a specific germ-layer (Activin-A and BMP4 for Mesoderm, Wnt and BMP4 for ectoderm and Activin A for Endoderm). Germ-layer specification is then followed by organoids maturation that occurs as a result of self-organization and tissue-specific growth factors leading to mature organoids: blood vessel and kidney (mesoderm), brain (ectoderm), liver, thyroid, intestine, stomach, lung (endoderm). b Adult stem cells (AdSCs) are tissue-specific therefore organoids specification and maturation is obtained through tissue-specific growth factors and self-organization (e.g. pancreas, endometrium, liver, prostate, stomach, intestine, lung). Bone morphogenetic protein 4 (BMP4); wingless-related integration site (Wnt)
Origin and tissue-specific growth factors for the generation of human organoids. Pluripotent Stem Cells (PSCs) and adult stem cells (AdSCs) are guided toward the maturation of a specific organoid by the introduction in culture of specific growth factors that activate (arrow up ↑, green) or repress (arrow down ↓, red) particular signaling pathways (Kim et al. 2020). Bone morphogenetic protein (BMP); epidermal growth factor (EGF); fibroblast growth factors (FGF); hepatocyte growth factor (HGF); insulin-like growth factor (IGF); microtubule associated protein kinase (MAPK); RHO-associated protein kinase (ROCK); transforming growth factor (TGF); vascular endothelial growth factor (VEGF); wingless-related integration site (Wnt)
Organoids technology takes advantage of the different characteristics of pluripotent stem cells (PSCs, both Embryonic Stem Cells and induced Pluripotent Stem Cells) and multipotent stem cells (Adult Stem Cells, AdSCs) to create 3D structures that could serve as in vitro models of different organs; therefore, offering the opportunity to observe important biological phenomena such as embryonic development and tissue regeneration and to develop personalized disease models through the building of patient-derived organoids (Lancaster and Huch 2019; Takahashi 2019; Schutgens and Clevers 2020; Zheng and Fu 2021).
On one hand, PSCs can differentiate toward all three germ layers (Endoderm, Ectoderm, Mesoderm) and are used for building more complex organoids useful for studying the embryonic development and are needed when the organ that has to be modeled is not easily accessible (e.g., the brain) (Brassard and Lutolf 2019; Liu et al. 2021; Yu et al. 2021). On the other hand, AdSCs, due to the more limited differentiation capability, are mostly used to generate organoids of their tissue of origin. AdSCs also offer the advantage of being isolated directly from patient’s biopsies thus making them a valuable tool for disease modeling and personalized medicine purposes. While the building of PSCs-derived organoids requires the reprogramming of somatic differentiated cells isolated from patients followed by expansion and differentiation, the use of AdSCs permits the production of healthy and diseased tissues in a shorter time: as a result, the latter allows a more manageable expansion of models from patients, potentially facilitating personalized medicine (Rossi et al. 2018; Lancaster and Huch 2019; Schutgens and Clevers 2020).
The generation of organoids requires also the addition of specific growth factors into the stem cell culture medium in the appropriate amount and spatiotemporal way. For instance, the step of germ-layer specification for PSCs is obtained through Activin A (Endoderm), Activin A and Bone Morphogenetic Protein 4 (BMP4, Mesoderm) and WNT + PBM4 (Ectoderm), which is then followed by a step in which tissue-specific growth factor cocktails and molecules activate particular signaling pathways, such as WNT and Fibroblast Growth Factors (FGF) (Yin et al. 2016; Lancaster and Huch 2019; Kim et al. 2020)(Figs. 1a, 2). The latter step allows the induction and maturation of organoids and is common also to the AdSCs-derived organoids maturation process (Figs. 1b, 2).
All steps of differentiation protocols aim at supplying stem cells with a range of biochemical and biophysical signals that mimic the in vivo stem cell niche, which is essential to create a good organoid model (Figs. 1, 2). This correlates with the concept that tissue and organ development, including cell specification, differentiation, survival, and proliferation, is heavily reliant on complex networks and coordination of cell-to-cell, and cell-Extracellular Matrix (ECM) interactions, as cooperative cell activity differs significantly from individual cell behavior (Dahl-Jensen and Grapin-Botton 2017).
The strict dependence of organoids formation and biochemical and biophysical environmental conditions is a crucial aspect that contributes deeply to the successful development of accurate models but also inevitably introduces a certain grade of randomness into organoids formation, resulting in heterogeneous morphology, size, and cellular composition (Hofer and Lutolf 2021). The concept of reproducibility in organoids research is one of the major obstacles for their scalability and full use in pre-clinical applications, hence fine-tuning the culture microenvironment is unquestionably essential for the advancement of this technology (Rossi et al. 2018; Lehmann et al. 2019; Brassard and Lutolf 2019; Hofer and Lutolf 2021). Indeed, there are hurdles that still need to be fully managed as low-maturation level, small size (not more than a few millimeters), morphological variability and lack of fundamental biological components like vascularization and immune system (Lancaster and Knoblich 2014; Shen 2018; Holloway et al. 2019; Brassard and Lutolf 2019; Zahmatkesh et al. 2021).
The delicate balance required to maintain homogeneous organoids cultures highlights the role of the environment in controlling the cellular polarization in a context-dependent manner (Brassard and Lutolf 2019). Thus, is now widely recognized that organoids formation is deeply influenced by small changes in the culture condition (Hofer and Lutolf 2021). Therefore, all methods used for organoids generation consist in the inclusion of stem cells in an environment characterized by specific biophysical and biochemical components (Fig. 3). These elements mimic the role of the structure as a well as of soluble biomolecules in the in vivo stem cell niche, allowing for better regulation of cellular growth and differentiation and, as a result, more physiological applicable model systems that can be translated into clinical practice (Hofer and Lutolf 2021).
Conventional methods for organoids generation. Schematization of the main steps required in the techniques most frequently used for organoids generation: ECM-scaffold-based, suspension culture, air–liquid interface, magnetic levitation and 3D bioprinting (grey column) with related examples of produced organoids (Blue column, references in the text). Schematic representation of method used for organoids generation: biological elements (cells) and microenvironment required for organoids maturation (biophysics and biochemical components). Pluripotent stem cells (PSCs); adult stem cells (AdSCs)
The commonest method currently used for the generation of organoids is the ECM-scaffold based (Shah and Singh 2017; Velasco et al. 2020). In this technique, organoids are generated by including stem cells in an environment consisting of a biophysical component, generally natural (Matrigel, Collagen, Alginate, Fibrin, Laminin) or synthetic (e.g., Polyethylene Glycol, PEG) hydrogels, and biochemical component, such as different types of soluble bioactive chemical/biological molecules (Sato et al. 2009; Kurmann et al. 2015; Workman et al. 2017; McCracken et al. 2017; Hohwieler et al. 2017; Shah and Singh 2017; Chen et al. 2017; Camp et al. 2017; Yan et al. 2018). Alternatively, organoids can be generated with the (i) suspension culture procedure accompanied by the use of spinner flasks or rotating bioreactors, which can be described as rotating cell culture systems (Nakano et al. 2012; Qian et al. 2018; Hoarau-Véchot et al. 2018; Przepiorski et al. 2018; Capowski et al. 2019; Velasco et al. 2020; Sander et al. 2020); (ii) Air–liquid interface (ALI), where stem cells are exposed to culture medium on one side and to air on the other for maximizing the oxygen and nutrient supply (Takasato et al. 2015; Neal et al. 2018; Choi et al. 2020; Lo et al. 2020; Esser et al. 2020; Gunti et al. 2021); (iii) Magnetic levitation, which poses its bases in tagging cells with magnetic nanoparticles and then exposing them to a magnetic field that levitates them to the liquid–air interface where they aggregate and generate ECM components (Desai et al. 2017; Tseng et al. 2018; Ferreira et al. 2019; Velasco et al. 2020); (iv) 3D bioprinting, which could allow controlling the spatial positioning of cells and other biological components such as growth factors and ECM structural components (Fig. 3)(Duelen et al. 2019; Reid et al. 2019; Sun et al. 2020; Kupfer et al. 2020; Rawal et al. 2021; Yang et al. 2021).
Organoids and mechanobiology
Mechanical forces and spatiotemporally coordinated cellular signaling patterning are now recognized as essential factors in tissues organization and acquisition of their functional adult state in vivo (Jansen et al. 2015; Weaver 2017; Mohammed et al. 2019; Argentati et al. 2019; Kim et al. 2021). The mechanical forces that regulate and act on the 3D adult tissue organization, are transmitted within the tissue by individual cells that are confined in the ECM (Humphrey et al. 2014; Stanton et al. 2019; Argentati et al. 2019; Kim et al. 2021). In this section, we will discuss the relevance of mechanobiology in organoids development. To be clear, the section starts with some notes on mechanobiology.
Pills of mechanobiology
Over the last two decades, evidence has accumulated demonstrating how the physico-chemical properties of the cellular microenvironment, as well as the physical forces exerted by cells and tissues, are critical in the regulation of physiological conditions (such as tissue development, repair, and homeostasis, cell motility, proliferation, metabolism and differentiation) (Mammoto and Ingber 2010; Morena et al. 2017, 2020; Argentati et al. 2018, 2019; Wolfenson et al. 2019) but also pathological states (Jansen et al. 2015; Jensen et al. 2015; Alcaraz et al. 2018; Kim et al. 2019; Lee et al. 2019; Argentati et al. 2019, 2020b; Hall et al. 2020). In both contexts, cells must adapt their behavior using their capability to sense the external physical forces—mechanosensing—and to transduce these forces into biochemical signals—mechanotransduction (Trubelja and Bao 2018; Martino et al. 2018; Argentati et al. 2019). Both mechanisms collect the activity of several intracellular and extracellular components (Table 1) that, working together in a spatial–temporal manner, transmit the signaling to the cell DNA and change the cell gene expression (Trubelja and Bao 2018; Martino et al. 2018; Argentati et al. 2019; Janota et al. 2020). The most known pathways include (i) integrins—ECM—Focal adhesion (FAs) complexes—cytoskeleton—nucleoskeleton proteins (Weinberg et al. 2017; Jansen et al. 2017; Morena et al. 2017; Martino et al. 2018; Luzi et al. 2020; Argentati et al. 2021); (ii) Adherens Junctions (AJs) complexes for cell–cell interaction—cytoskeleton—nucleoskeleton proteins (Morena et al. 2017; Martino et al. 2018; Yap et al. 2018; Liebman et al. 2020). The overall interconnection also influences the behavior of neighboring cells and can remodel constantly the ECM environment through synthesis, degradation, and chemical modification processes (Humphrey et al. 2014; Stanton et al. 2019; Argentati et al. 2019).
In addition, several studies have identified molecular components involved in the mechano-sensing and—transduction processes, which respond to various mechanical forces such as compression (cells contract as a result of compressive forces applied from the outside to the center of cells)(Takemoto et al. 2015; Vining and Mooney 2017; Argentati et al. 2019), tension (external stimuli that stretch cells in opposite directions, resulting in cell elongation)(Spadaro et al. 2017; Martino et al. 2018; Rossy et al. 2018; Argentati et al. 2019), hydrostatic pressure (force exercised by the surrounding fluid to cells membranes, with non-directional nature influencing microtubule stability of cell cytoskeleton) (Becquart et al. 2016; Hadi et al. 2018; Pattappa et al. 2019), and fluid shear stress (two opposing forces applied tangentially to a cell's surface, causing changes in cell morphology and adhesion properties) (Becquart et al. 2016; Alfieri et al. 2019; Argentati et al. 2019) that in turn lead to the deformation and regulation of particular cellular environment properties including elasticity (the ability of an object to revert to its original shape and size after a force has been removed)(Grady et al. 2016; Argentati et al. 2019), stiffness (the ability of an object to resist deformation after being subjected to a force) (Islam et al. 2017; Argentati et al. 2019; Janmey et al. 2020) and viscoelasticity (an object’s elastic and viscous properties that contrast deformation)(Wang et al. 2016a; Argentati et al. 2019; Chaudhuri et al. 2020). (Table 1). These processes are likely activated when stem cells generate organoids (Bayir et al. 2019; Hofer and Lutolf 2021).
Mechanical forces involved in stem cell-derived organoids formation
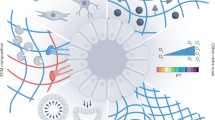
The engineering of the organoid microenvironment focuses on controlling diverse mechanical properties such as topography, porosity, permeability, stiffness, shape, and elasticity (Bayir et al. 2019). The combination of all these properties creates a specific microenvironment characterized by a particular set of forces that are exerted on cells indirectly via the ECM, allowing them to mechanosense and respond to these forces when forming an organoid (Fig. 4) (Dahl-Jensen and Grapin-Botton 2017; Park et al. 2019).
Mechanical forces and organoids formation. Schematic representation of the involvement of different mechanical and physical forces (shear stress, tension, compression, hydrostatic pressure) and environmental properties (stiffness and elasticity) in the main steps of organoids formation: a Stem cells are included in an environment characterized by specific chemical and structural components; b Different mechanical forces and environmental properties influence stem cells specification and c 3D self-organization; d All these forces and properties guide the maturation of organoids and e lead to the formation of specific organoids type
The identification of the appropriate pattern of forces that have to be present in culture is fundamental for steering stem cells toward the right differentiation state (Vining and Mooney 2017). Performing experiments could fully elucidate how mechanics affect particular cells or tissues in vivo, in fact several studies clarified how substrates with different mechanical properties allowed lineage-specific differentiation of stem cells. For example, matrix elasticity regulates the differentiation of Mesenchymal Stem Cells (MSCs) with the general concept that rigidity is associated with chondrogenic/osteogenic lineages and softer matrices induce neuronal or fat differentiation (Engler et al. 2006; Huebsch et al. 2010; Khetan et al. 2013; Vining and Mooney 2017; Romani et al. 2021).
Indeed, stiffness is a decisive parameter for mimicking the stem cells’ niche and it can be tuned using synthetic matrices which, in this way, offer the possibility of investigating its effect on organoids formation (Gjorevski et al. 2016). About this, new mechanical refined materials such as complex hydrogels with tunable architecture and composition that offer the possibility of precisely control the orientation of functional groups showed that the regulation of matrix viscoelasticity and gel degradability is of particular importance for a successful organoid formation and culture (Cruz-Acuña et al. 2017; Chaudhuri et al. 2020).
As far as understanding the sensing of mechanical stimuli by organoids is concerned, the clarification of how forces exactly influence organoids formation is even more difficult because they are a more complex model (compared to 2D cultures)(Chan et al. 2017) in which cells establish interactions among them and the external ECM; however, several studies explored this issue (Park et al. 2019; Bayir et al. 2019).
In this regard, in a recent study, the laboratory of H. Clevers investigated the role of matrix stiffness on the behavior of Intestinal Stem Cells (ISCs). In this work, they evidenced how Intestinal Stem Cells cultured on a stiff matrix underwent expansion enhancement, but when grown on a soft matrix differentiated and formed organoids (Gjorevski et al. 2016). In particular, first, they cultured ISCs in PEG hydrogels functionalized with the RGD (Arg-Gly-Asp) peptide and observed that ISCs expanded on the matrix with intermediate stiffness and did not on softer ones (1.3 vs 300 Pa), and afterward they used hybrid PEG hydrogels constituted by a mechanically static and a mechanically dynamic PEG to control over time the gel’s stiffness: when functionalized with RGD and laminin-111, organoids were generated only when gel stiffness was about 190 Pa and Yes-associated protein (YAP) activation was greater in these softening matrices. This study, therefore, shed light on the mechanistic role of the 3D microenvironment (Gjorevski et al. 2016).
Acknowledged the importance of mechanical forces in embryogenesis and organogenesis, the control of the biophysical microenvironment answer to the need of enhancing the reliability of organoid models. For this reason, it is now becoming clear that it is necessary to build culture systems in which is possible to produce biomechanical cues that are as physiological as possible. Recent advancement in this field is the synergic combination of organoids and organ-on-a-chip (OOC) technology: while organoids have the advantage of following self-organization, OOC offers the possibility of precisely regulate the cellular microenvironment to replicate the physiological environmental conditions (Park et al. 2019; Zheng et al. 2021). There are several OOC available on the market that employ dynamic biomechanical stimulation and that can be used to develop complex 3D tissues like spheroids, organoids, and tissues interfaces (Thompson et al. 2020).
For example, Lee et al. implemented peristaltic fluid flow in human stomach organoids; therefore, introducing contraction and stretching to mimic gastric contractions, which enabled the construction of a more solid and physiologically relevant model amenable for disease modeling and drug screening (Lee et al. 2018). To do so, human gastric organoids (GOs) generated from hPSCs were cultured in a 3D-printed device equipped with micropipettes connected to a peristaltic pump filled with FITC-dextran: following the fluorescent fluid flow, they observed a regular distribution of luminal fluid overtime and demonstrated the feasibility of GOs long-term culturing associated to nutrient and therapeutic agents delivery (Lee et al. 2018).
Berger et al. enhanced the vitality and differentiation of Midbrain organoids using a fluidic system that generated continuous laminar fluid flow (Berger et al. 2018). They compared a new milli-fluidic culture technique with the orbital shaker (commonly used for brain organoids generation) and observed that it allowed a better differentiation of Neuroepithelial Stem Cells to midbrain Dopaminergic neurons and a reduction of the inner area of cell death: interestingly, this work highlighted that different fluid dynamics have distinct effects on organoids development suggesting that the resulting diverse mechanical stimuli are involved in their homeostasis (Berger et al. 2018).
Another promising result was obtained by Tao et al. that generated iPSCs-derived Pancreas organoids in a microfluidic system that improved their viability and organ-specific functionality, like insulin secretion stimulated by glucose and higher Ca2+ flux (Tao et al. 2019). In accordance with the study previously proposed, this work showed that the culture of organoids under perfused conditions highlights the role of biomimetic mechanical signals in improving the functionality and maturation of islet organoids (Tao et al. 2019).
In another study, Homan et al. exploited shear stress generated with a milli-fluidic system and co-culture with endothelial cells to greatly improve the maturation of Kidney organoids managing to enhance vasculature and their tubular and glomerular compartments (Homan et al. 2019). In particular, they determined the effect of fluidic shear stress by culturing hPSCs in a chip with controlled fluid flow and observed that the vascular network formation was greatly improved under high fluidic shear stress condition compared to low, in the order of fivefold increase, indicating that shear stress is a significant cue for the vascularization of kidney organoids in vitro as it is associated to the endogenous upregulation of the vascular endothelial growth factor (Homan et al. 2019).
Conclusion
In this mini review, we have discussed recent key findings on the development of organoid technology (Fig. 3). In particular, we have highlighted the relevance of the environment as an active counterpart on inducing stem cells toward the generation of a specific organoid, describing the role of exogenous soluble bioactive molecules and foremost the role of the environmental physical components, and the way in which both mimic the structure and function of the stem cell niche. The role of mechanical forces has been demonstrated to significantly orchestrate the interaction of the cells with the ECM or with neighboring cells and how these interconnections are fundamental for cell functions. These roles have been confirmed also in organoids formation. Of note, to date, there are different medical applications of organoid mechanobiology-based technology such as novel drug screening, regenerative medicine application, molecular research (Fig. 5).
In this regard, many studies focused on organoids mechanobiology are ongoing and will help to elucidate the mechanism behind the biophysical aspects of organoid cultures. For instance, the European Project “Mechanoids” (Grant agreement ID: 797,621, H2020-EU.1.3.2.) aims at manipulating the mechanobiology of healthy Gut and Colorectal Cancer organoids to assess their role in disease and development processes (HORIZON 2020a) The characterization of organoids mechanobiology will be useful also for disease modeling, as planned in the project “ROMB” (Grant agreement ID: 850,691, H2020-EU.1.1.) where Retina organoids mechanobiology will be investigated to model Alzheimer’s Disease and will shed light on mechanically related neuronal diseases (HORIZON 2020b). In conclusion, despite the challenges that must be addressed, considering the advantages of ongoing technology development, organoid technology holds great promise in research and in the developing clinical translational strategies.
References
Alcaraz J, Otero J, Jorba I, Navajas D (2018) Bidirectional mechanobiology between cells and their local extracellular matrix probed by atomic force microscopy. Semin Cell Dev Biol 73:71–81
Alfieri R, Vassalli M, Viti F (2019) Flow-induced mechanotransduction in skeletal cells. Biophys Rev 11:729–743
Andrikakou P, Vickraman K, Arora H (2016) On the behaviour of lung tissue under tension and compression. Sci Rep 6:1–10. https://doi.org/10.1038/srep36642
Argentati C, Morena F, Bazzucchi M et al (2018) Adipose stem cell translational applications: from bench-to-bedside. Int J Mol Sci 19:3475. https://doi.org/10.3390/ijms19113475
Argentati C, Morena F, Tortorella I et al (2019) Insight into mechanobiology: how stem cells feel mechanical forces and orchestrate biological functions. Int J Mol Sci 20:5337. https://doi.org/10.3390/ijms20215337
Argentati C, Tortorella I, Bazzucchi M et al (2020a) Harnessing the potential of stem cells for disease modeling: progress and promises. J Pers Med 10:8. https://doi.org/10.3390/jpm10010008
Argentati C, Tortorella I, Bazzucchi M et al (2020b) The other side of alzheimer’s disease: influence of metabolic disorder features for novel diagnostic biomarkers. J Pers Med 10:2–36
Argentati C, Morena F, Fontana C et al (2021) Functionalized silica star-shaped nanoparticles and human mesenchymal stem cells: an in vitro model. Nanomaterials 11:779. https://doi.org/10.3390/nano11030779
Atherton P, Stutchbury B, Jethwa D, Ballestrem C (2016) Mechanosensitive components of integrin adhesions: role of vinculin. Exp Cell Res 343:21–27
Bayir E, Sendemir A, Missirlis YF (2019) Mechanobiology of cells and cell systems, such as organoids. Biophys Rev 11:721–728
Becquart P, Cruel M, Hoc T et al (2016) Human mesenchymal stem cell responses to hydrostatic pressure and shear stress. Eur Cells Mater 31:160–173. https://doi.org/10.22203/eCM.v031a11
Bell S, Terentjev EM (2017) Focal adhesion kinase: the reversible molecular mechanosensor. Biophys J 112:2439–2450. https://doi.org/10.1016/j.bpj.2017.04.048
Berger E, Magliaro C, Paczia N et al (2018) Millifluidic culture improves human midbrain organoid vitality and differentiation. Lab Chip 18:3172–3183. https://doi.org/10.1039/c8lc00206a
Brassard JA, Lutolf MP (2019) Engineering stem cell self-organization to build better organoids. Cell Stem Cell 24:860–876
Brouhard GJ, Rice LM (2018) Microtubule dynamics: an interplay of biochemistry and mechanics. Nat Rev Mol Cell Biol 19:451–463
Camp JG, Sekine K, Gerber T et al (2017) Multilineage communication regulates human liver bud development from pluripotency. Nature 546:533–538. https://doi.org/10.1038/nature22796
Capowski EE, Samimi K, Mayerl SJ et al (2019) Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Dev 146:171686. https://doi.org/10.1242/dev.171686
Chan CJ, Heisenberg CP, Hiiragi T (2017) Coordination of morphogenesis and cell-fate specification in development. Curr Biol 27:R1024–R1035
Charrier EE, Janmey PA (2016) Mechanical properties of intermediate filament proteins, In: methods in enzymology, Academic Press Inc., pp 35–57
Chaudhuri O, Cooper-White J, Janmey PA et al (2020) Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584:535–546
Chen YW, Huang SX, De Carvalho ALRT et al (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat Cell Biol 19:542–549. https://doi.org/10.1038/ncb3510
Chen L, Jiang F, Qiao Y et al (2018) Nucleoskeletal stiffness regulates stem cell migration and differentiation through lamin A/C. J Cell Physiol 233:5112–5118
Choi KYG, Wu BC, Lee AHY et al (2020) Utilizing organoid and air-liquid interface models as a screening method in the development of new host defense peptides. Front Cell Infect Microbiol 10:228
Chooi WH, Chan BP (2016) Compression loading-induced stress responses in intervertebral disc cells encapsulated in 3D collagen constructs. Sci Rep 6:1–14. https://doi.org/10.1038/srep26449
Cocciolone AJ, Hawes JZ, Staiculescu MC et al (2018) Elastin, arterial mechanics, and cardiovascular disease. Am J Physiol Hear Circ Physiol 315:H189–H205
Cruz-Acuña R, Quirós M, Farkas AE et al (2017) Synthetic hydrogels for human intestinal organoid generation and colonic wound repair. Nat Cell Biol 19:1326–1335. https://doi.org/10.1038/ncb3632
Dahl-Jensen S, Grapin-Botton A (2017) The physics of organoids: a biophysical approach to understanding organogenesis. Dev 144:946–951. https://doi.org/10.1242/dev.143693
Demaio L, Chang YS, Gardner TW et al (2001) Shear stress regulates occludin content and phosphorylation. Am J Physiol Hear Circ Physiol 281:H105–H113. https://doi.org/10.1152/ajpheart.2001.281.1.h105
Desai P, Tseng H, Souza G (2017) Assembly of hepatocyte spheroids using magnetic 3D cell culture for CYP450 inhibition/induction. Int J Mol Sci 18:1085. https://doi.org/10.3390/ijms18051085
Di Russo J, Luik A, Yousif L et al (2017) Endothelial basement membrane laminin 511 is essential for shear stress response. EMBO J 36:183–201. https://doi.org/10.15252/embj.201694756
Duelen R, Corvelyn M, Tortorella I et al (2019) Medicinal biotechnology for disease modeling, clinical therapy, and drug discovery and development, In: Introduction to biotech entrepreneurship: from idea to business, Springer International Publishing, pp 89–128
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689. https://doi.org/10.1016/j.cell.2006.06.044
Esser LK, Branchi V, Leonardelli S et al (2020) Cultivation of clear cell renal cell carcinoma patient-derived organoids in an air-liquid interface system as a tool for studying individualized therapy. Front Oncol 10:1775. https://doi.org/10.3389/fonc.2020.01775
Fan YL, Zhao HC, Li B et al (2019) Mechanical roles of F-Actin in the differentiation of stem cells: a review. ACS Biomater Sci Eng 5:3788–3801
Fernandez A, Bautista M, Pinaud F (2021) Emerin oligomerization and nucleoskeletal coupling at the nuclear envelope regulate nuclear mechanics against stress. bioRxiv 2021.02.12.429834. https://doi.org/10.1101/2021.02.12.429834
Ferreira JN, Hasan R, Urkasemsin G et al (2019) A magnetic three-dimensional levitated primary cell culture system for the development of secretory salivary gland-like organoids. J Tissue Eng Regen Med 13:495–508. https://doi.org/10.1002/term.2809
Fujita K, Ohmachi M, Ikezaki K et al (2019) Direct visualization of human myosin II force generation using DNA origami-based thick filaments. Commun Biol 2:1–11. https://doi.org/10.1038/s42003-019-0683-0
Galkin VE, Orlova A, Egelman EH (2012) Actin filaments as tension sensors. Curr Biol 22:R96
Gjorevski N, Sachs N, Manfrin A et al (2016) Designer matrices for intestinal stem cell and organoid culture. Nature 539:560–564. https://doi.org/10.1038/nature20168
Grady ME, Composto RJ, Eckmann DM (2016) Cell elasticity with altered cytoskeletal architectures across multiple cell types. J Mech Behav Biomed Mater 61:197–207. https://doi.org/10.1016/j.jmbbm.2016.01.022
Gunti S, Hoke ATK, Vu KP, London NR (2021) Organoid and spheroid tumor models: techniques and applications. Cancers (basel) 13:1–18
Gupta M, Doss B, Lim CT et al (2016) Single cell rigidity sensing: a complex relationship between focal adhesion dynamics and large-scale actin cytoskeleton remodeling. Cell Adhes Migr 10:554–567
Haas AJ, Zihni C, Ruppel A et al (2020) Interplay between extracellular matrix stiffness and JAM-A regulates mechanical load on ZO-1 and tight junction assembly. Cell Rep 32:107924. https://doi.org/10.1016/j.celrep.2020.107924
Hadi A, Rastgoo A, Haghighipour N, Bolhassani A (2018) Numerical modelling of a spheroid living cell membrane under hydrostatic pressure. J Stat Mech Theory Exp 2018:83501. https://doi.org/10.1088/1742-5468/aad369
Hall CM, Moeendarbary E, Sheridan GK (2020) Mechanobiology of the brain in ageing and Alzheimer’s disease. Eur J Neurosci 00:1–28. https://doi.org/10.1111/ejn.14766
Hamant O, Inoue D, Bouchez D et al (2019) Are microtubules tension sensors? Nat Commun 10:1–12. https://doi.org/10.1038/s41467-019-10207-y
Herrero-Galán E, Martínez-Martín I, Alegre-Cebollada J (2019) Redox regulation of protein nanomechanics in health and disease: lessons from titin. Redox Biol 21:101074
Hoarau-Véchot J, Rafii A, Touboul C, Pasquier J (2018) Halfway between 2D and animal models: are 3D cultures the ideal tool to study cancer-microenvironment interactions? Int J Mol Sci 19:181. https://doi.org/10.3390/ijms19010181
Hofer M, Lutolf MP (2021) Engineering organoids. Nat Rev Mater 6:402–420. https://doi.org/10.1038/s41578-021-00279-y
Hohwieler M, Illing A, Hermann PC et al (2017) Human pluripotent stem cell-derived acinar/ductal organoids generate human pancreas upon orthotopic transplantation and allow disease modelling. Gut 66:473–486. https://doi.org/10.1136/gutjnl-2016-312423
Holloway EM, Capeling MM, Spence JR (2019) Biologically inspired approaches to enhance human organoid complexity. Development 146(8):dev166173. https://doi.org/10.1242/dev.166173
Homan KA, Gupta N, Kroll KT et al (2019) Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16:255–262. https://doi.org/10.1038/s41592-019-0325-y
HORIZON (2020a) Probing and controlling the three-dimensional organoid mechanobiology. MECHANOIDS Project, CORDIS, European Commission. https://cordis.europa.eu/project/id/797621. Accessed 22 Apr 2021
HORIZON (2020b) Retina Organoid Mechanobiology. ROMB Project, CORDIS, European Commission. https://cordis.europa.eu/project/id/850691. Accessed 17 Apr 2021
Huebsch N, Arany PR, Mao AS et al (2010) Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater 9:518–526. https://doi.org/10.1038/nmat2732
Humphrey JD, Dufresne ER, Schwartz MA (2014) Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15:802–812
Ikawa K, Sugimura K (2018) AIP1 and cofilin ensure a resistance to tissue tension and promote directional cell rearrangement. Nat Commun 9:1–14. https://doi.org/10.1038/s41467-018-05605-7
Imanaka-Yoshida K, Aoki H (2014) Tenascin-C and mechanotransduction in the development and diseases of cardiovascular system. Front Physiol 5: https://doi.org/10.3389/fphys.2014.00283
Islam M, Brink H, Blanche S et al (2017) Microfluidic sorting of cells by viability based on differences in cell stiffness. Sci Rep 7:1–12. https://doi.org/10.1038/s41598-017-01807-z
Jang I, Beningo KA (2019) Integrins, CAFs and mechanical forces in the progression of cancer. Cancers (Basel). 11
Janmey PA, Fletcher DA, Reinhart-King CA (2020) Stiffness sensing by cells. Physiol Rev 100:695–724. https://doi.org/10.1152/physrev.00013.2019
Janota CS, Calero-Cuenca FJ, Gomes ER (2020) The role of the cell nucleus in mechanotransduction. Curr Opin Cell Biol 63:204–211
Jansen KA, Donato DM, Balcioglu HE et al (2015) A guide to mechanobiology: where biology and physics meet. Biochim Biophys Acta - Mol Cell Res 1853:3043–3052
Jansen KA, Atherton P, Ballestrem C (2017) Mechanotransduction at the cell-matrix interface. Semin Cell Dev Biol 71:75–83
Jensen MH, Morris EJ, Weitz DA (2015) Mechanics and dynamics of reconstituted cytoskeletal systems. Biochim Biophys Acta Mol Cell Res 1853:3038–3042. https://doi.org/10.1016/j.bbamcr.2015.06.013
Kechagia JZ, Ivaska J, Roca-Cusachs P (2019) Integrins as biomechanical sensors of the microenvironment. Nat Rev Mol Cell Biol 20:457–473
Khetan S, Guvendiren M, Legant WR et al (2013) Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater 12:458–465. https://doi.org/10.1038/nmat3586
Kim J-K, Shin YJ, Ha LJ et al (2019) Unraveling the Mechanobiology of the immune system. Adv Healthc Mater 8:1801332. https://doi.org/10.1002/adhm.201801332
Kim J, Koo BK, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584
Kim S, Uroz M, Bays JL, Chen CS (2021) Harnessing mechanobiology for tissue engineering. Dev Cell 56:180–191
Koushki N, Ghagre A, Srivastava LK, et al (2020) Lamin A redistribution mediated by nuclear deformation determines dynamic localization of YAP. bioRxiv 2020.03.19.998708. https://doi.org/10.1101/2020.03.19.998708
Kumar A, Ouyang M, Van den Dries K et al (2016) Talin tension sensor reveals novel features of focal adhesion force transmission and mechanosensitivity. J Cell Biol 213:371–383. https://doi.org/10.1083/jcb.201510012
Kupfer ME, Lin WH, Ravikumar V et al (2020) In situ expansion, differentiation, and electromechanical coupling of human cardiac muscle in a 3D bioprinted, chambered organoid. Circ Res 127:207–224. https://doi.org/10.1161/CIRCRESAHA.119.316155
Kurmann AA, Serra M, Hawkins F et al (2015) Regeneration of thyroid function by transplantation of differentiated pluripotent stem cells. Cell Stem Cell 17:527–542. https://doi.org/10.1016/j.stem.2015.09.004
LaCroix AS, Lynch AD, Berginski ME, Hoffman BD (2018) Tunable molecular tension sensors reveal extension-based control of vinculin loading. Elife 7:33927. https://doi.org/10.7554/eLife.33927
Lancaster MA, Huch M (2019) Disease modelling in human organoids. DMM Dis Model Mech 12:dmm039347. https://doi.org/10.1242/dmm.039347
Lancaster MA, Knoblich JA (2014) Organogenesis in a dish: Modeling development and disease using organoid technologies. Science 345:1247125–1247125. https://doi.org/10.1126/science.1247125
Lee KK, McCauley HA, Broda TR et al (2018) Human stomach-on-a-chip with luminal flow and peristaltic-like motility. Lab Chip 18:3079–3085. https://doi.org/10.1039/c8lc00910d
Lee G, Han SB, Lee JH et al (2019) Cancer mechanobiology: microenvironmental sensing and metastasis. ACS Biomater Sci Eng 5:3735–3752
Lehmann R, Lee CM, Shugart EC et al (2019) Human organoids: a new dimension in cell biology. Mol Biol Cell 30:1129–1137. https://doi.org/10.1091/mbc.E19-03-0135
Liebman C, McColloch A, Rabiei M, et al (2020) Mechanics of the cell: Interaction mechanisms and mechanobiological models, In: current topics in membranes. Academic Press Inc., pp 143–184
Liu X, Tan JP, Schröder J et al (2021) Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature 591:627. https://doi.org/10.1038/s41586-021-03372-y
Lo YH, Karlsson K, Kuo CJ (2020) Applications of organoids for cancer biology and precision medicine. Nat Cancer 1:761–773
Lou SS, Kennard AS, Koslover EF et al (2021) Elastic wrinkling of keratocyte lamellipodia driven by myosin-induced contractile stress. Biophys J. https://doi.org/10.1016/j.bpj.2021.02.022
Luzi F, Tortorella I, Di Michele A et al (2020) Novel nanocomposite PLA films with lignin/zinc oxide hybrids: design, characterization interaction with mesenchymal stem cells. Nanomaterials 10:2176. https://doi.org/10.3390/nano10112176
Mammoto T, Ingber DE (2010) Mechanical control of tissue and organ development. Development 137:1407–1420
Martino F, Perestrelo AR, Vinarský V et al (2018) Cellular mechanotransduction: from tension to function. Front Physiol 9:824
McCracken KW, Aihara E, Martin B et al (2017) Wnt/β-catenin promotes gastric fundus specification in mice and humans. Nature 541:182–187. https://doi.org/10.1038/nature21021
Meacci G, Wolfenson H, Liu S et al (2016) α-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol Biol Cell 27:3471–3479. https://doi.org/10.1091/mbc.E16-02-0107
Mezawa M, Pinto VI, Kazembe MP et al (2016) Filamin A regulates the organization and remodeling of the pericellular collagen matrix. FASEB J 30:3613–3627. https://doi.org/10.1096/fj.201600354RR
Mohammed D, Versaevel M, Bruyère C et al (2019) Innovative tools for mechanobiology: unraveling outside-in and inside-out mechanotransduction. Front Bioeng Biotechnol 7:162
Morena F, Armentano I, Montanucci P et al (2017) Design of a nanocomposite substrate inducing adult stem cell assembly and progression toward an Epiblast-like or Primitive Endoderm-like phenotype via mechanotransduction. Biomaterials 144:211–229. https://doi.org/10.1016/j.biomaterials.2017.08.015
Morena F, Argentati C, Soccio M et al (2020) Unpatterned bioactive poly(Butylene 1,4-cyclohexanedicarboxylate)-based film fast induced neuronal-like differentiation of human bone marrow-mesenchymal stem cells. Int J Mol Sci 21:1–23. https://doi.org/10.3390/ijms21239274
Nakano T, Ando S, Takata N et al (2012) Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10:771–785. https://doi.org/10.1016/j.stem.2012.05.009
Nakasaki M, Hwang Y, Xie Y et al (2015) The matrix protein Fibulin-5 is at the interface of tissue stiffness and inflammation in fibrosis. Nat Commun 6:1–11. https://doi.org/10.1038/ncomms9574
Neal JT, Li X, Zhu J et al (2018) Organoid modeling of the tumor immune microenvironment. Cell 175:1972-1988.e16. https://doi.org/10.1016/j.cell.2018.11.021
Omachi T, Ichikawa T, Kimura Y et al (2017) Vinculin association with actin cytoskeleton is necessary for stiffness-dependent regulation of vinculin behavior. PLoS ONE 12:e0175324. https://doi.org/10.1371/journal.pone.0175324
Pannekoek WJ, de Rooij J, Gloerich M (2019) Force transduction by cadherin adhesions in morphogenesis. F1000Research 8
Park SE, Georgescu A, Huh D (2019) Organoids-on-a-chip. Science 364:960–965
Pattappa G, Zellner J, Johnstone B et al (2019) Cells under pressure - the relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. Eur Cell Mater 37:360–381
Przepiorski A, Sander V, Tran T et al (2018) A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Rep 11:470–484. https://doi.org/10.1016/j.stemcr.2018.06.018
Qian X, Jacob F, Song MM et al (2018) Generation of human brain region—specific organoids using a miniaturized spinning bioreactor. Nat Protoc 13:565–580. https://doi.org/10.1038/nprot.2017.152
Rawal P, Tripathi DM, Ramakrishna S, Kaur S (2021) Prospects for 3D bioprinting of organoids. Bio Design Manuf 1:3
Reid JA, Palmer XL, Mollica PA et al (2019) A 3D bioprinter platform for mechanistic analysis of tumoroids and chimeric mammary organoids. Sci Rep 9:1–10. https://doi.org/10.1038/s41598-019-43922-z
Romani P, Valcarcel-Jimenez L, Frezza C, Dupont S (2021) Crosstalk between mechanotransduction and metabolism. Nat Rev Mol Cell Biol 22:22–38
Rossi G, Manfrin A, Lutolf MP (2018) Progress and potential in organoid research. Nat Rev Genet 19:671–687
Rossy J, Laufer JM, Legler DF (2018) Role of mechanotransduction and tension in t cell function. Front Immunol 9:2638
Saini K, Kumar N (2015) Mechanical response of collagen molecule under hydrostatic compression. Mater Sci Eng C 49:720–726. https://doi.org/10.1016/j.msec.2015.01.032
Sander V, Przepiorski A, Crunk AE et al (2020) Protocol for large-scale production of kidney organoids from human pluripotent stem cells. STAR Protoc 1:100150. https://doi.org/10.1016/j.xpro.2020.100150
Sarpal R, Yan V, Kazakova L et al (2019) Role of α-Catenin and its mechanosensing properties in regulating Hippo/YAP-dependent tissue growth. PLoS Genet 15:e1008454. https://doi.org/10.1371/journal.pgen.1008454
Sato T, Vries RG, Snippert HJ et al (2009) Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459:262–265. https://doi.org/10.1038/nature07935
Schrenk S, Cenzi C, Bertalot T et al (2018) Structural and functional failure of fibrillin-1 in human diseases (Review). Int J Mol Med 41:1213–1223
Schutgens F, Clevers H (2020) Human organoids: tools for understanding biology and treating diseases. Annu Rev Pathol Mech Dis 15:211–234. https://doi.org/10.1146/annurev-pathmechdis-012419-032611
Shah SB, Singh A (2017) Cellular self-assembly and biomaterials-based organoid models of development and diseases. Acta Biomater 53:29–45
Shen H (2018) Organoids have opened avenues into investigating numerous diseases. But how well do they mimic the real thing? Proc Natl Acad Sci U S A 115:3507–3509. https://doi.org/10.1073/pnas.1803647115
Sheng X, Sheng Y, Liu Y et al (2018) Effects of FSS on the expression and localization of the core proteins in two Wnt signaling pathways, and their association with ciliogenesis. Int J Mol Med 42:1809–1818. https://doi.org/10.3892/ijmm.2018.3758
Spadaro D, Le S, Laroche T et al (2017) Tension-dependent stretching activates ZO-1 to control the junctional localization of its interactors. Curr Biol 27:3783-3795.e8. https://doi.org/10.1016/j.cub.2017.11.014
Stanton AE, Tong X, Yang F (2019) Extracellular matrix type modulates mechanotransduction of stem cells. Acta Biomater 96:310–320. https://doi.org/10.1016/j.actbio.2019.06.048
Sun W, Starly B, Daly AC et al (2020) The bioprinting roadmap. Biofabrication 12:022002
Takahashi T (2019) Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol 59:447–462. https://doi.org/10.1146/annurev-pharmtox-010818-021108
Takasato M, Er PX, Chiu HS et al (2015) Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526:564–568. https://doi.org/10.1038/nature15695
Takemoto K, Ishihara S, Mizutani T et al (2015) Compressive stress induces dephosphorylation of the myosin regulatory light chain via RhoA phosphorylation by the adenylyl cyclase/protein kinase a signaling pathway. PLoS ONE 10:e0117937. https://doi.org/10.1371/journal.pone.0117937
Tao T, Wang Y, Chen W et al (2019) Engineering human islet organoids from iPSCs using an organ-on-chip platform. Lab Chip 19:948–958. https://doi.org/10.1039/C8LC01298A
Thompson CL, Fu S, Knight MM, Thorpe SD (2020) Mechanical stimulation: a crucial element of organ-on-chip models. Front Bioeng Biotechnol 8:602646
Trubelja A, Bao G (2018) Molecular mechanisms of mechanosensing and mechanotransduction in living cells. Extrem Mech Lett 20:91–98
Tseng H, Daquinag AC, Souza GR, Kolonin MG (2018) Three-dimensional magnetic levitation culture system simulating white adipose tissue, In: methods in molecular biology, Humana Press Inc., pp 147–154
Velasco V, Shariati SA, Esfandyarpour R (2020) Microtechnology-based methods for organoid models. Microsystems Nanoeng 6:1–13
Vining KH, Mooney DJ (2017) Mechanical forces direct stem cell behaviour in development and regeneration. Nat Rev Mol Cell Biol 18:728–742
Wang Z, Golob MJ, Chesler NC (2016a) Viscoelastic properties of cardiovascular tissues. In: Viscoelastic and viscoplastic materials. InTech
Wang K, Seo BR, Fischbach C et al (2016b) Fibronectin mechanobiology regulates tumorigenesis. Cel Mol Bioeng 9:1–11. https://doi.org/10.1007/s12195-015-0417-4
Weaver VM (2017) Cell and tissue mechanics: the new cell biology frontier. Mol Biol Cell 28:1815–1818
Wei F, Xu X, Zhang C et al (2020) Stress fiber anisotropy contributes to force-mode dependent chromatin stretching and gene upregulation in living cells. Nat Commun 11:1–12. https://doi.org/10.1038/s41467-020-18584-5
Weinberg SH, Mair DB, Lemmon CA (2017) Mechanotransduction dynamics at the cell-matrix interface. Biophys J 112:1962–1974. https://doi.org/10.1016/j.bpj.2017.02.027
Wiesolek HL, Bui TM, Lee JJ et al (2020) Intercellular adhesion Molecule 1 functions as an efferocytosis receptor in inflammatory macrophages. Am J Pathol 190:874–885. https://doi.org/10.1016/j.ajpath.2019.12.006
Willer MK, Carroll CW (2017) Substrate stiffness-dependent regulation of the SRF-Mkl1 co-activator complex requires the inner nuclear membrane protein Emerin. J Cell Sci 130:2111–2118. https://doi.org/10.1242/jcs.197517
Wolfenson H, Yang B, Sheetz MP (2019) Steps in mechanotransduction pathways that control cell morphology. Annu Rev Physiol 81:585–605
Workman MJ, Mahe MM, Trisno S et al (2017) Engineered human pluripotent-stem-cell-derived intestinal tissues with a functional enteric nervous system. Nat Med 23:49–59. https://doi.org/10.1038/nm.4233
Yan HHN, Siu HC, Law S et al (2018) A comprehensive human gastric cancer organoid biobank captures tumor subtype heterogeneity and enables therapeutic screening. Cell Stem Cell 23:882-897.e11. https://doi.org/10.1016/j.stem.2018.09.016
Yang H, Sun L, Pang Y et al (2021) Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 70:567–574. https://doi.org/10.1136/gutjnl-2019-319960
Yap AS, Duszyc K, Viasnoff V (2018) Mechanosensing and mechanotransduction at cell–cell junctions. Cold Spring Harb Perspect Biol 10:a028761. https://doi.org/10.1101/cshperspect.a028761
Yin X, Mead BE, Safaee H et al (2016) Engineering stem cell organoids. Cell Stem Cell 18:25–38
Yu L, Wei Y, Duan J et al (2021) Blastocyst-like structures generated from human pluripotent stem cells. Nature 591:620–626. https://doi.org/10.1038/s41586-021-03356-y
Zahmatkesh E, Khoshdel-Rad N, Mirzaei H et al (2021) Evolution of organoid technology: lessons learnt in Co-Culture systems from developmental biology. Dev Biol 475:37–53
Zheng Y, Fu J (2021) First complete model of the human embryo. Nature 591:531–532. https://doi.org/10.1038/d41586-021-00581-3
Zheng F, Xiao Y, Liu H, et al (2021) Patient‐specific organoid and organ‐on‐a‐chip: 3D cell‐culture meets 3D printing and numerical simulation. Adv Biol 2000024. https://doi.org/10.1002/adbi.202000024
Zhou DW, Lee TT, Weng S et al (2017) Effects of substrate stiffness and actomyosin contractility on coupling between force transmission and vinculin-paxillin recruitment at single focal adhesions. Mol Biol Cell 28:1901–1911. https://doi.org/10.1091/mbc.E17-02-0116
Zimmermann D, Kovar DR (2019) Feeling the force: formin’s role in mechanotransduction. Curr Opin Cell Biol 56:130–140
Funding
Open access funding provided by Università degli Studi di Perugia within the CRUI-CARE Agreement. This research received no external funding.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: Nanoengineering for Mechanobiology.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tortorella, I., Argentati, C., Emiliani, C. et al. The role of physical cues in the development of stem cell-derived organoids. Eur Biophys J 51, 105–117 (2022). https://doi.org/10.1007/s00249-021-01551-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00249-021-01551-3