Abstract
Bacillus species appearas the most attractive plant growth-promoting rhizobacteria (PGPR) and alternative to synthetic chemical pesticides. The present study examined the antagonistic potential of spore forming-Bacilli isolated from organic farm soil samples of Allahabad, India. Eighty-seven Bacillus strains were isolated and characterized based on their morphological, plant growth promoting traits and molecular characteristics. The diversity analysis used 16S-rDNA, BOX-element, and enterobacterial repetitive intergenic consensus. Two strains, PR30 and PR32, later identified as Bacillus sp., exhibited potent in vitro antagonistic activity against Ralstonia solanaceorum. These isolates produced copious amounts of multiple PGP traits, such as indole-3-acetic acid (40.0 and 54.5 μg/mL), phosphate solubilization index (PSI) (4.4 and 5.3), ammonia, siderophore (3 and 4 cm), and 1-aminocyclopropane-1-carboxylate deaminase (8.1and 9.2 μM/mg//h) and hydrogen cyanide. These isolates were subjected to the antibiotic sensitivity test. The two potent isolates based on the higher antagonistic and the best plant growth-promoting ability were selected for plant growth-promoting response studies in tomatoe, broccoli, and chickpea. In the pot study, Bacillus subtilis (PR30 and PR31) showed significant improvement in seed germination (27–34%), root length (20–50%), shoot length (20–40%), vigor index (50–75%), carotenoid content (0.543–1.733), and lycopene content (2.333–2.646 mg/100 g) in tomato, broccoli, and chickpea. The present study demonstrated the production of multiple plant growth-promoting traits by the isolates and their potential as effective bioinoculants for plant growth promotion and biocontrol of phytopathogens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacteria of the genus Bacillus are soil-borne, endospore-forming, and stress-resistant bacteria from the phylum Firmicutes. They are ubiquitously present in many ecological conditions [1]. Gram-positive Bacillus species are the most promising plant growth-promoting rhizobacteria (PGPR), ecologically sound, and economically viable alternative to the pesticide usage in agriculture [2, 3]. These bacterial strains colonize the crop rhizosphere, efficiently suppress phytopathogens, and promote plant growth. Several researchers have reported the diversity, phylogeny, production, and secretion of degradative enzymes to combat phytopathogens [4], the production of a wide array of secondary metabolites and antibiotics [5, 6], and defense mechanisms like plant-induced systemic resistance [7, 8]. Some of the species of Bacillusaredescribed as endophytes that can promote plant growth using varied mechanisms, including colonization in roots, enlargement of root density, solubilization of minerals, enhanced nutrient uptake, and induced defense responses against abiotic and biotic factors [2, 9, 10]. Moreover, researchers employ Bacillus isolates that exhibit multifarious potentials such as phosphorus solubilization [11], production of indole-3-acetic acid (IAA) [12], siderophore [13], and 1-aminocyclopropane-1-carboxylate deaminase (ACCD) activity [14]. Bacillus species arewidely used biocontrol agents against various phytopathogens as commercially developed formulations are available [2, 15, 16].Commercially available forms of some Bacillus species include phytostimulants [17], biopesticides, and biofertilizers [18]. It has been widely used on various plants, including tobacco, soybean, cucumber, maize, rice, and watermelon [18,19,20,21].
Bacterial wilt caused by Ralsatonia solanacearum is a quarantine phytopathogen responsible for devastating agricultural losses worldwide [22]. It is a soil-borne phytopathogen that infects various commercial crops and can survive for long periods in the soil. During favorable conditions, the dormant bacterium is activated, enters through primary and secondary roots, and colonizes the host plants’ xylem vessels, leading to a lethal wilt disease. This disease causes enormous losses from the time of sowing till maturity. The disease is characterized by the appearance of yellow-colored leaves and light brown-colored lesions on the shoot androot, leading toreduced crop yield [22].
The present study evaluatedthe diversity, plant growth-promoting ability, and antagonistic activity of Bacillus sp. against R. solanacearum in tomato, broccoli, and chickpea.
Materials and Methods
Soil Sample
Ten grams of each sandy-loam soil sample were collected from the rhizosphere soil of tomato plants in Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS) Model Organic Farm (SMOF) Allahabad, India, (25° 24′ 42" N, 81° 50′ 56" E) [23, 24] (Fig. 1). Briefly, a rhizosphere soil sample (1 g) was transferred to 9 mL of sterilized phosphate buffer saline (PBS; 10 mL in 100 mL flask; pH 7.2) for 30 min, heated at 80 °C for 20 min in a water bath. The soil suspensions were serially diluted (10–3 to 10–5), and 0.1 mL of this suspension was spread on Nutrient Agar (NA) plates comprising methyl red (0.2%). The plates were incubated for 24 h at 30 °C. Single bacterial colonies were streaked onto fresh NA plates. Endospore staining was performed as described by Hamouda et al. [25].
Screening of Antagonistic Bacillus Sp.
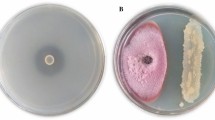
Seven selected Bacillus isolates were used for in vitro antagonistic activity using disc diffusion against R. solanacearum [26]. The growth inhibition of the pathogen was measured as the percent growth inhibition (PGI) using the following formula -
where C = measure of control group growth and T = measure of treatment group.
The test was repeated 3 times with 5 independent replications.
Molecular Identification of Bacillus Isolates
The standard microbiological protocols were followed for morphological and biochemical characterization of isolates. DNA extraction, partial 16S rRNA gene amplification, PCR product purification, and subsequent sequencing analysis of Bacillus isolates were performed as previously described [27]. The 16S rRNAgenewas amplified using universal primers, PF [5′-TGGCTCAGATTGAACGCTGGCGG-3′] and PR [5′-TGGCTCAGATTGAACGCTGGCGG-3′], and the PCR products were sequenced on ABI3100 Genetic Analyzer. The amplified sequences were run in the BLASTnprogram and compared with the NCBI database.
Using the multiple sequence alignment tool Clustal W, consensus sequences of the 16S rRNA gene from Bacillus isolates and reference sequences obtained from Genbank were aligned, using MEGA version 5 to contruct a phylogenetic analysis [28]. The unweighted pair group technique with the arithmetic mean (UPGMA) approach was used to conduct the analysis. The greatest composite likelihood approach was used to calculate the evolutionary distances, and the evolutionary history was constructed using the neighbor-joining method [29] with 2000 bootstrap replications, and the internal branches’ robustness was evaluated.
Molecular identification of Bacillus isolates was performed using rep-PCR with the help of BOXA1R ERIC-1R and ERIC-2F primers [28]. The PCR reaction mixture (25 μL) containing 5 × Gitschier buffer, 0.25 μM of primer, 50 ng DNA template, 1 U of Taq DNA polymerase (Bangalore Genie, India), and 2 mM MgCl2 was amplified on thermal Cycler (Biorad, CA, USA) and the amplified fragments were separated by agarose gel electrophoresis with the help of aDNA ladder of 100 bp to 3 kb.
Screening for Plant Growth-Promoting Traits
Indole Acetic Acid Production
Indole acetic acid (IAA) production by the isolates was screened in Luria Bertani (LB) medium containing 1 g/L tryptophan [30].
Phosphate Solubilizing Activity
The inorganic phosphate solubilizing (PS) activities of bacterial isolates were perceived using the National Botanical Research Institute’s phosphate growth medium (NBRIP) agar medium at 37 ± 2 °C for 7 days. The abilityof the isolates to solubilize inorganic phosphate was calculated as a solubilization index (SI) using the following formula [31].
Production of Ammonia
Isolates were screened for ammonia (NH3) production in peptone water (10 mL) inoculated and incubated for 48–72 h. Following the incubation, Nestler’s reagent (0.5 mL) was added, and the tubes were observed to color change from brown to yellow [32].
Siderophore Production
Screening of isolates for siderophore (SD) production was performed on Chrome Azurol agar (CAS) medium [33] at 30 °C. The isolates were grown on CAS agar medium at 30 °C for 48 h and observed for color change of medium from blue to orange/golden.
1-Aminocyclopropane-1-Carboxylate Deaminase Activity
According to the available protocol, isolates were screened for 1-aminocyclopropane- 1-carboxylate deaminase (ACCD) activity [30]. The ACCD activity was defined as the amount of α-keto-butyrate produced per mg of protein per h.
Hydrogen Cyanide
Hydrogen cyanide (HCN) production by Bacillus isolates was estimated according to Lork’s protocol [34].
Susceptibility to Antibiotics
Bacillus isolates were tested according to the method of Bauer [35] for its resistance to various antibiotics (Table 4). All assays were performed three times with five replications.
Plant Growth Promotion Studies
In Vitro Study
The seeds of different vegetable crops such as tomato (variety-Lycopersicon; NTL-186), broccoli (variety-PalamSamridhi), and chickpea (variety- Pusa 256) were surface-sterilized with ethanol (70%) for 2 min and washed three times with sterile distilled water. The following treatments were applied.
T0- control (no bacterial inoculation),
T1 = B. subtilis PR30,
T2 = B. subtilis PR31.
The surface-sterilized seeds were transferred to bacterial suspension (104 cfu/mL), kept for 60 min, placed on germination paper, and maintained for 15 days at 25 °C [24]. Control (T0) seeds without bacterial inoculation were used for comparison. Treated and control seeds were also checked for the root and shoot elongation pattern under in vitro conditions. All the treatments were performed in triplicates, and the average of triplicates was considered.
Percent Germination
The percent seed germination was calculated as follows [36].
Germination Index
The germination index (GI) was calculated according to the Association of Official Seed Analysts AOSA [37] using the following formula–
Vigor Index
Vigor index was calculated according to Abdul and Anderson [38] and with the help of the following formula.
Green-House Pot Experiment
Plant Seeds and Treatment
The bioefficacy of Bacillus isolates (PR30 and PR31) was evaluated under a greenhouse pot experiment at Sam Higginbottom University of Agriculture, Technology and Sciences (SHUATS), Allahabad, India. Surface sterilized broccoli, tomato, and chickpea seeds were treated with endospore suspension of Bacillus isolates (~ 108 cells mL−1). Treated seeds were sown in plastic pots containing autoclaved field soil and watered daily. Control seeds were not treated with bacterial culture. Five seed pot−1 and 10 pot treatment−1 were maintained. The treatments for the bioefficacy experiment were scheduled as (1) B. subtilis PR30, (2) B. subtilis PR31, (3) and Control. Plant growth parameters were measured 30, 60, and 90 days after sowing (DAS).
Measurement of Plant Growth Parameters
Ten seedlings were harvested, root length, shoot length, fresh weight, and dry weight (mg/plant) were measured.The plant height (cm) was measured from the ground level to the growing tip of the main shoot, and the average height was calculated in cm. One plant was randomly selected from each pot, and the number of branches of each plant was measured and averaged.Flowers from three plants were counted and averaged. All the fruits from three selected plants from each replication of all the treatments were counted. All the fresh fruits from three selected plants from each replication of all the treatments were weighed after picking. Each replication’s average fresh fruit weight per plant (g) was recorded and subjected to statistical analysis. The diameter of the curd of three plants was measured at the widest circumference in cm, and the average diameter per curd from each pot was calculated. The Head or bud of three selected plants was weighted on electrical balance in g, and the average was found to give the bud weight per plant. The fresh weights of the three selected plants were recorded in each pot, and the average fresh weight was calculated. This calculated value was assumed as the average weight of the rest of the remaining plant per pot. The plants used to measure dry weight were also subjected to dry weight analysis. The plants were dried for 5–6 h at a temperature of 50–60 °C. The dry weights of all randomly selected plants in each pot were added, and the average was calculated.
Biochemical Parameters
Chlorophyll, Carotenoid, and Lycopene Content
Chlorophyll a, b, and carotenoid content were determined according to Arnon's method [39]. The absorbance of the resulting solution was read at 663, 645, and 480 nm for chlorophyll a, b, and carotenoids. The extraction and estimation of lycopene content was performed according to the method of Butnariuand Giuchici [40]. The absorbance of supernatant containing lycopene was read in a spectrophotometer at 472 nm. The total lycopene content was measured as lycopene mg per 100 g of fruit tissues.
where E = extinction coefficient; W = weight (g).
Statistical Analysis
All the experiments were performed in triplicates, and the average valueswere calculated. The standard errors were calculated for all mean values and subjected to ANOVA followed by DMRT. The BOX and ERIC-PCR product cluster analysis was based on the binary matrix, presence (1), and absence (0) of the band for each strain. Principle component analysis (PCA) was performed using the XLSTAT software.
Results
Isolation and In Vitro Assessment of Bacillus Isolates
A total 7 potent Bacillus isolates, (PR30, PR31, PR35, PR38, PR42, PR45, and PR55) were obtained from diverse soilsamples. These isolates showed antagonistic activity against Ralstonia solanacearum (Fig. 2) (Table 1).
Values are the mean of three replicates. According to Duncan’s multiple range test, different letters in superscript are significantly different (p ≤ 0.05).
Morphological and Biochemical Characterization of Strains
The Bacillusisolates were first characterized by their morphological and biochemical attributes. The isolateswere motile, spore-forming rods, forming white to creamy-white colonies, endospore former, tolerated pH (5–9), temperature (10–45 °C), and NaCl (0–10%) (Table 2).
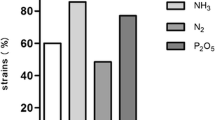
They hydrolyzed starch and produced catalase. Three strains (PR30, PR42, and PR45) produced H2S, ammonia,siderophores IAA, HCN, and ACCD (Table 3). All strains showed intrinsic antibiotic resistance to variable levels (Table 4).
Identification of antagonistic Bacillus isolates
Seven Bacillusisolates were recognized as the active antagonists. Their phylogenetic distribution and 16S rRNA gene sequence individualities are presented in Table 5. The similarity values (≥ 97%) confirmed that all isolates (PR30, PR31, PR33, PR38, PR42, PR45, and PR48) belong to the genus Bacillus subtilis. The un-rooted phylogenetic tree (Fig. 3) presented the genotypic relationship of the isolates, wherever all the bacterial isolates were clustered into two major clades. The 16S-rRNA data showed that PR31and PR35 (B. subtilis) isolate was more diverse than other strains and grouped into distinct clusters.
Phylogenetic tree based on the 16S rRNA gene sequence of potent Bacillus isolates were generated from organic farm soil samples based on the 16S rRNA gene sequence. Evolutionary distances were calculated using the “neighbor-joining” algorithm, based on a bootstrap analysis of 2000 replicates (values on branches denote % ofbootstrap support)
ERIC and BOX-PCR Analysis
ERIC's complex fingerprint patterns produced 81 polymorphic bands of variable range (250–3000 bp) (Fig. 4a). Principal component analysis (PCA) based on the first and second coordinates showed a maximum Eigen value of 4.642 and minimum value of 0.095 with a percent variation of 66.31 and 14.30, respectively (Fig. 4b). Observation of the PCA analysis revealed that four isolates (PR30, PR31, PR35, and PR55) formed a major cluster (cluster I). Three isolates were classifiedin cluster II (PR38, PR42, and PR45).
The BOX-PCR banding pattern of all seven strains displayed 102 fragments in the 250–4000 bp (Fig. 4c). The results of the PCA analysis are based on the first and second coordinates and showed a maximum Eigen value of 3.053 and minimum value of 0.162 with a percentage variation of 50.05 and 13.90%, respectively (Fig. 4d). PCA analysis revealed that four isolates (PR30, PR31, PR38, and PR55) formed a major cluster (cluster II), and three isolates (PR35, PR42, and PR45) were classifiedin cluster I.
Evaluation for Bioefficacy
Based on antagonistic activity and PGP traits, two isolates (PR30 and PR31) were selected for plant growth parameters of different vegetable crops under in vitro and greenhouse conditions. The Bacillus isolates (PR30 and PR31) induced a higher percentage (85–96%) of germination of seed compared to control seeds (Table 6).
The Bacillus spp. isolates (PR30 and PR31) showed the best responses for plant growth promotion under greenhouse conditions. The efficacy of selected Bacillus isolates varied to induce germination, vigor index, and root and shoot length in tomato, broccoli, and chickpea (Table 6). Tomato seedling treatment with B. subtilis PR30 and B. subtilis PR31 significantly (p ≤ 0.05) enhanced seed germination (27%), root length (20–50%), shoot length (20–40%), fresh weight, and dry biomass (50–75%) compared to untreated (Fig. 5a) (Tables 6, 7). In the case of broccoli, a significant improvement in seed germination (15–24%), root length (40–60%), shoot length (50–60%), fresh weight, and dry biomass (50–60%) was evident over the control (Fig. 5b) (Tables 7 and 8). While this inoculation also improved seed germination (50–60%), root length (40–50%), shoot length (20–40%), and vigor index (50–80%) in chickpea (Fig. 5c). Bacillus subtilis PR30 and PR31 isolates significantly improved the chlorophyll and carotenoid content in tomato, chickpea, and broccoli, whereas lycopene was considerably enhanced in tomato plants (Table 8).
Discussion
Increasing agricultural productivity with limited cultivable land is the biggest challenge to growers around the globe. It is necessary to improve agricultural productivity to nourish and feed the growing world population. Crop yield and productivity can be enhanced in two ways: by increasing crop productivity through fertilizers or biofertilizers and by preventing crop losses caused by phytopathogens. Using PGPR that possesses the dual potential of plant growthpromotion and biocontrol is expected to play this dual role [24].
The diverse potential of Bacillus spp. makes it a promising plant growth-promoting rhizobacterium and BCA in various crops. Inoculation of crops with Bacillus spp. promotesseed germination, seedling vigor, leaf index, root and shoot growth, and photosynthetic ability. The plant growth promotion due to Bacillus spp. inoculation is due to the production of various PGP substances [2, 14]. Inhibition of phytopathogens results from the secretion of a wide range of antagonistic substances [17, 26]. Members of the Bacillus genus produce multiple PGP traits, such as phytohormones, and they help in nutrient mobilization (iron, P, etc.) [7, 14], which improves the growth of inoculated crop plants. Bacillus spp. is one of the major biological control agents (BCA) and antagonistic soil bacterium [21, 26]. Bacillus sp. produces various antagonistic substances, such as hydrogen cyanide, siderophore, and hydrolytic enzymes to inhibit the growth of phytopathogens.
Developing biofertilizers/formulation strategies using spore-forming Bacillusbioagents is an emerging area in crop protection. A total of 87 Bacillus strains were isolated from organic farm soil samples and examined by performing a disc diffusion approach to raise the possibility of using antagonistic bacteria asBCAs against Ralstonia solanacearum. Using this strategy, seven potent B. subtilis strains (PR30, PR31, PR35, PR38, PR42, PR45, and PR55) were specifically selected as an effective biocontrol agent against R. solanacearum. Similar strategies have been used effectively to isolate potential BCAs, such as Bacillus strains exhibiting antimicrobial activity towards phytopathogens [41].
Molecular identification based on 16S rRNA gene sequences of Bacillus isolates indicates phylogenetic clustering between bacteria at inter- and intra-species levels [42]. At the same time, the present study perceived that identification based on 16S rRNA gene sequences is limited and incapable of distinguishing along with bacterial strains. Consequently, polyphasic gene-based fingerprinting tools (BOX-PCR and ERIC-PCR] were used to discriminate intra-species unevenness between the bacterial strains. The results presented that isolates could not be distinguished by partial 16S rRNA gene sequence were different regarding ERIC and BOX-PCR patterns. Besides, these tools help separate the ecologically diverse Bacillus strains into a distinct group, which is otherwise tricky through 16S rRNA gene analysis [23].
Seedling treatment among isolates significantly suppressed the pathogen growth in in vitro conditions and enhanced plant height and biomass compared to control. Inhibition of phytopathogens is attributed to various antagonistic substances,viz.siderophore, hydrogen cyanide, other volatile compounds, and a wide range of antibiotics. Siderophore-producing PGPR prevents iron nutrition and, hence, the growth of phytopathogens [43]. HCN is a volatile organic compound (VOC) synthesized by a wide range of PGPR. Many bacterial genera, including Bacillus, can produce HCN [6, 20]. HCN exerts a potent toxic effect on many phytopathogens, forming stable complexes with Cu2+, Fe2+, and Mn2+ and causing disruption in protein functions [25, 44] (Reference?). It also inhibits electron transport and disrupts the energy supply to the cell, leading to living organisms’ death. Besides their biocontrol ability to produce HCN and antibiotics, Bacillus species produce phytohormones, increase uptake of phosphate and iron, produce ammonia, and protect cells from oxidative damage by producing catalase enzyme. Rahman et al. [43] reported the inhibition of Agrobacterium tumefaciensby HCN-producing Bacillus megaterium strain CtST3.5.
Many PGPR produce IAA, a crucial phytohormone associated with root elongation and initiation.Plants provide tryptophan to PGPR to synthesize IAA, an essential phytohormone for plant growth promotion. Bacillus spp. produced copious amounts of IAA, and this IAA has promoted root ramification.
Phosphorus is one of the vital elements for plant growth and development. It is regarded as a limiting nutrient for plant growth as it is usually present in insoluble forms. P solubilizing PGPR can potentially solubilize P and make it available forplant growth promotion [45].
Liu et al. [14] also suggested that the B. amyloliquefaciens strain displays maximum in vitro inhibitory activity towards multiple plant pathogens. Sudha et al. [45] reported the production of volatile compounds in Streptomyces rochei that inhibited the growth of sorghum pathogen. Sayyed and Patel [46] presented siderophore production in Alcaligenesfaecalis and found that this siderophoregenic culture inhibits the growth of a wide range of fungal phytopathogens. They found more antifungal activity in siderophoregenic culture than in a chemical fungicide. The present study also demonstrated that B. subtilis PR31 colonized more frequently than other test strains in tomato and broccoli rhizosphere, while B. subtilis PR30 was assessed more in chickpea. These findings justify the previous biocontrol reports that emphasize the proficient colonization of BCAs in the host rhizosphere,which is expected to enhance plant growth promotion and disease management.
Biocontrol efficacy of Bacillus spp. has been confirmed in the greenhouse and field conditions and at the post-harvest stage for fruit diseases [47]. It has been established primarily to resist gram-negative bacteria in vitro and under controlled conditions and to reduce diseases caused by these pathogens. A single strain can act against numerous bacterial pathogens. For example, B. velezensis LS69 has been shown to display antibacterial activities against Erwinia carotovora and Ralstonia solanacearum [43, 48]. Production of plant growth-promoting traits (PGPT) is the characteristic feature of all PGPR. These PGPT promote plant growth through direct mechanisms as green biostimulants [2, 49]. PGPR promotes plant growth through an indirect mechanism, such as the production of antibiotics [43] and the production of hydrolytic enzymes. The induction of resistance in plants and production of siderophore [46, 50] and phosphate solubilizing ability in different cultures of PGPR isolated from the rhizosphere have been reported. Kapadia et al. [51] reported the production of multiple plant growth-promoting traits in Bacillus sp., Klebsiella variiocola, and Mesorhizobium sp., respectively, and found that this multipotent culture improves growth in wheat and maize. The Bacillus sutilis isolates identified and used in the present study possessed all the plant growth promoting traits and hence are ideal candidates for biological control of R. solanacearum.
The production of ammonia is one of the major traits of PGPR that helps promote plant growth. The production of ACCD in PGPR is one of the best mechanisms involved in plant growth promotion under oxidative stress. PGPR lowers the ACC level in root exudates, decreasing the concentration of ethylene in the plant roots and thus helps in root length for better absorption of nutrients.Through antioxidants and other mechanisms, PGPR induces resistance to protect crop plants, thus helping plant growth [47, 48, 52,53,54].
Bacillus spp. suppressing the development of a wide range of phytopathogens while promoting plant growth in various crops can make this culture a multipotent PGPR for sustainable plant disease management and an eco-friendly biocontrol agent [2].
The present study successfully screened the Bacillus strains such as B. subtilis PR30 and B. subtilis PR31 associated with tomato rhizosphere that could stimulate growth in various crops (tomato, chickpea, and broccoli). These strains are identified as potent antagonists to suppress the growth of R. solaniunder in vitro conditions. They can be used in integrated disease management of tomato root rot and damping off. The combined studies, comprising biochemical and molecular technologies, are essential to select indigenous antagonistic Bacillus strains that can be used in combinations of other strains under different environmental conditions (greenhouse and field conditions to obtain resistance against pathogens in various crops). Without the extraordinary effect of genetic resistance in tomato cultivars, these biocontrols may be a potential candidate for handling vascular wilt infection and minimizing losses in enhanced fruit quality and yield. However, additional investigations are required to conclude the efficiency of these strains under diverse cultivar varieties and locations to understand the interaction behavior with the pathogen, host plants, and soil factors.
Data Availability
No datasets were generated or analysed during the current study.
Abbreviations
- ACCD:
-
1-Aminocyclopropane-1-carboxylate deaminase
- GI:
-
Germination index
- HCN:
-
Hydrogen cyanide
- IAA:
-
Indole-3-acetic acid
- MOF:
-
Model Organic Farm
- NA:
-
Nutrient agar
- PGI:
-
Percent growth inhibition
- PGP:
-
Plant growth promoting
- PGPR:
-
Plant growth-promoting rhizobacteria
- PSI:
-
Phosphate solubilization index
- SD:
-
Siderophore
- SHUATS:
-
Higginbottom University of Agriculture, Technology and Sciences
References
Gurr GM, You M (2016) Conservation biological control of pests in the molecular era: new opportunities to address old constraints. Front Plant Sci 6:1255. https://doi.org/10.3389/fpls.2015.01255
Sagar A, Yadav SS, Sayyed RZ, Sharma S, Ramteke PW (2022) Bacillus subtilis: a multifarious plant growth promoter, biocontrol agent, and bioalleviator of abiotic stress,” in Bacilli in agrobiotechnology; Plant Stress Tolerance, Bioremediation, and Bioprospecting, Islam M T, Rahman M, and Pandey P (eds), Springer, Berlin 561–580. https://doi.org/10.1007/978-3-30-85465-2_24
Radhakrishnan R, Hashem A, Abd_Allah EF (2017) Bacillus: a biological tool for crop improvement through bio-molecular changes in adverse environments Frontiers in Physiol 8. https://doi.org/10.3389/fphys201700667
Nithyapriya S, Lalitha S, Sayyed RZ, Reddy MS, Dailin DJ, Enshasy HA, Suriani NL, Herlambang S (2021) Production, purification, and characterization of Bacillibactin siderophore of Bacillus subtilis and its application for improvement in plant growth and oil content in Sesame, Sustainability, 13, 5394 https://doi.org/10.3390/su13105394
Kiesewalter HT, Lozano-Andrade CN, Wibowo M, Strube ML, Maróti G, Snyder D, Jørgensen TS, Larsen TO, Cooper VS, Weber T, Kovács ÁT (2021) Genomic and chemical diversity of Bacillus subtilis secondary metabolites against plant pathogenic fungi.mSystems 6:e00770–20 https://doi.org/10.1128/mSystems00770-20
Xiong, ZR, Cobo, M, Whittal, RM, Snyder, AB, and Worobo, RW (2022) Purification and c by Bacillus velezensis isolated from raw honey PLoS ONE 17[4]: e0266470. https://doi.org/10.1371/journal.pone.0266470ss
Ayaz M, Ali Q, Farzand A, Khan A, Ling H, Gao X (2021) Nematicidal volatiles from Bacillus atrophaeus GBSC56 promote growth and stimulate induced systemic resistance in tomato against Meloidogyne incognita. Int J Mol Sci 22(9):5049. https://doi.org/10.3390/ijms22095049
Ali SAM, Sayyed RZ, Mir MI, Khan MY, Hameeda B, Alkhanani MF, Haque S, Mohammad Al Tawaha AR, Poczai P (2022) Induction of systemic resistance in maize and antibiofilm activity of surfactin from Bacillus velezensis MS20. Front Microbiol 9(13):879739. https://doi.org/10.3389/fmicb2022879739
Akhtar N, Ilyas N Yasmin H, Sayyed RZ, Hasnain Z, Elsayed EA, El Enshasy H (2021) Role of Bacillus cereus in improving the growth and phytoextractability of Brassica nigra[L] K Koch in Chromium Contaminated Soil Molecules, 26,1569 https://doi.org/10.3390/molecules26061569
Manasa M, Ravinder P, Gopalakrishnan S, Srinivas V, Sayyed RZ, El Enshasy HA, Yahayu M, KeeZuan AT, Kassem HS, Hameeda B (2021) Co-Inoculation of Bacillus spp for growth promotion and iron fortification in sorghum, Sustainability, 13;12091 .https://doi.org/10.3390/su132112091
Mažylyte R, Kaziuniene J, Orola L, Valkovska V, Lastauskiene E, Gegeckas A (2022) Phosphate solubilizing microorganism Bacillussp mvy-004 and its significance for biomineral fertilizers’ development in Agrobiotechnology Biology, 11;254 https://doi.org/10.3390/biology11020254
Sarmiento-López LG, López-Meyer M, Maldonado-Mendoza IE, Quiroz-Figueroa FR, Sepúlveda-Jiménez G, and Rodríguez-Monroy M (20220 Production of indole-3-acetic acid by Bacillus circulans E9 in a low-cost medium in a bioreactor, J BiosciBioeng 20:S1389–1723;22:00085–8. https://doi.org/10.1016/jjbiosc202203007
Ghazy N, El-Nahrawy S (2021) Siderophore production by Bacillus subtilis MF497446 and Pseudomonas koreensis MG209738 and their efficacy in controlling Cephalosporium maydis in maize plant Arch Microbiol 203;1195–1209. https://doi.org/10.1007/s00203-020-02113-5
Liu H, Chen G-H, Sun J-J, Chen S, Fang Y, and Ren J-H (2022) Isolation, characterization, and tea growth-promoting analysis of jw-cz2, a bacterium with 1- aminocyclopropane-1-carboxylic acid deaminase activity isolated from the rhizosphere soils of tea plants. Front Microbiol 13:792876. https://doi.org/10.3389/fmicb.2022.792876
Fujimoto A, Augusto F, Fill TP, Moretto RK, Kupper KC (2022) Biocontrol of Phyllostictacitricarpa by Bacillusspp: biological and chemical aspects of the microbial interaction World J Microbiol Biotechnol 10;38[3]:53. https://doi.org/10.1007/s11274-614021-03214-z
Gohil RB, Raval VH, Panchal RR and Rajput KN (2022) Plant growth-promoting activity of bacillus sp pg-8 isolated from fermented panchagavya and its effect on the growth of Arachis hypogea, Front Agron, 4:805454. https://doi.org/10.3389/fagro.2022.805454
Luo L, Zhao C, Wang E, Raza A, Yin C (2022) Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: an overview for its mechanisms. Microbiol Res 259:127016
Bueno CB, dos Santos RM, de Souza Buzo F, de Andrade da Silva MSR, Rigobelo EC (2022) Effects of chemical fertilization and microbial inoculum on Bacillus subtilis colonization in soybean and maize plants. Front. Microbiol. 13:901157. https://doi.org/10.3389/fmicb.2022.901157
Saechow S, Thammasittirong A, Kittakoop P, Prachya S, Thammasittirong SNR (2018) Antagonistic activity against dirty panicle rice fungal pathogens and plant growth-promoting activity of Bacillus amyloliquefaciens BAS23. J Microbiol Biotechn 28(9):1527–1535
Wu YC, Zhou JY, Li CG, Ma Y (2019) Antifungal and plant growth promotion activity of volatile organic compounds produced by Bacillus amyloliquefaciens. MicrobiologyOpen 8(8):e00813
Jiao R, Cai Y, He P, Munir S, Li X, Wu Y, Wang J, Xia M, He P, Wang G, Yang H, Karunarathna SC, Xie Y, He Y (2021) Bacillus amyloliquefaciens YN201732 produces lipopeptides with promising biocontrol activity against the fungal pathogen Erysiphe cichoracearum. Front Cell Infect Microbiol 11:598999
Yuan W, Ruan S, Qi G, Wang R, Zhao X (2022) Plant growth-promoting and antibacterial activities of cultivable bacteria alive in tobacco field against Ralstoniasolanacearum. Environ Microbiol 24(3):1411–1429. https://doi.org/10.1111/1462-2920.15868
Sagar A, Debbarma V, Abraham T, Shukla PK, and Ramteke PW (2017) Functional diversity of soil bacteria from organic agroecosystem, Int J Curr Microbiol App Sci 6: 3500–3518 e https://doi.org/10.20546/ijcmas.2017.612.408
Sagar A, Sayyed RZ, Ramteke PW, Sharma S, Marraiki N, Elgorban AM, Syed A (2020) ACC deaminase and antioxidant enzymes producing halophilic Enterobactersp PR14 promotes the growth of rice and millets under salinity stress. Physiol and MolBiol of Plants 26:1847–1854. https://doi.org/10.1007/s12298-020-00852-9
Hamouda T, Shih AY, Baker JR (2002) A rapid staining technique for the detection of the initiation of germination of bacterial spores. Lett Appl Microbiol 34:86–90
Ahmed W, Zhou G, Yang J et al (2022) Bacillus amyloliquefaciens WS-10 as a potential plant growth promoter and biocontrol agent for bacterial wilt disease of flue-cured tobacco. Egypt J Biol Pest Control, 32:25. https://doi.org/10.1186/s41938-022-00527-5
Rademaker JLW, Louws FJ, de Bruijn FJ (1998) Characterization of the diversity of ecologically important microbes by Rep-PCR genomic fingerprinting. In: Akkermans ADL, van Elsas JD, de Bruijn FJ (eds) Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, pp 127–134. https://doi.org/10.1007/978-1-4020-2177-0_306
Rai S, Kashyap PL, Kumar S, Srivastava AK, Ramteke PW (2016) Comparative analysis of microsatellites in five different antagonistic Trichoderma species for diversity assessment. World J Microbiol Biotechnol 32:8. https://doi.org/10.1007/s11274-015-1964-5
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425. https://doi.org/10.1093/oxfordjournals.molbev.a040454
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15. https://doi.org/10.1034/j1399-3054200300086
Abbas S, Javed MT, Shahid M, Hussain I, Haider MZ, Chaudhary HJ, Tanwir K, Maqsood A (2020) Acinetobactersp SG-5 inoculation alleviates cadmium toxicity in differentially Cd tolerant maize cultivars as deciphered by improved physiobiochemical attributes, antioxidants, and nutrient physiology. Plant PhysiolBiochem 155:815–827. https://doi.org/10.1016/j.plaphy.2020.08.024
Cappuccino JC, Sherman N (1999) Microbiology. A Laboratory Manual 3rd ed Benjamin/Cummings Publication Co, New York, USA, pp 125–179
Patel PR, Shaikh SS, Sayyed RZ (2018) Modified chrome azurol S method for detection and estimation of siderophores having an affinity for metal ions other than iron. Envi Sustain 1(1):81–87. https://doi.org/10.1007/s42398-018-0005-3
Lorck H (1948) Production of hydrocyanic acid by bacteria Physiol. Planta 1(2):142–146. https://doi.org/10.1111/j.1399-3054.1948.tb07118.x
Bauer AW, Kirby WMM, Sherries JC, Turck M (1966) Antibiotic susceptibility testing by a standardized single disk method. Amer J Clin Pathol 45:493–496
Pandey S, Kumari A, Gupta KJ (2017) Isolation of physiologically active and intact mitochondria from chickpea in plant respiration and internal oxygen. Methods in Mol Biol 1670:77–85. https://doi.org/10.1007/978-1-4939-7292-0_9
Association of Official Seed Analysts [AOSA] (1983) Seed Vigor Testing Handbook. AOSA, Ithaca, NY, USA. (Contribution to the Handbook on Seed Testing, 32)
Abdul-Baki AA, Anderson JD (1973) Vigor determination in soybean seed by multiple criteria. Crop Sci 13:630–633. https://doi.org/10.2135/cropsci1973.0011183X001300060013x
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenol oxidase in Betavulgaris. Plant Physiol 25:1–15. https://doi.org/10.1104/pp.24.1.1
Butnariu MV, Giuchici CV (2011) The use of some nanoemulsions based on aqueous propolis and lycopene extract in the skin’s protective mechanisms against UVA radiation. J Nanobiotechnol 9:3. https://doi.org/10.1186/1477-3155-9-3
Hallaj-Nezhadi S, Hamdipour R, Shahrvirani M et al (2022) Antimicrobial activity of Bacillus sp isolated strains of wild honey BMC Complement Med Ther 22:78 https://doi.org/10.1186/s12906-022-03551-y
Kumbar B, Mahmood R, Narasimhappa NS (2017) Identification and molecular diversity analysis of Bacillus subtilis from soils of Western Ghats of Karnataka using 16S rRNA bacterial universal primers. Int J Pure App Biosci 5(20):541–548. https://doi.org/10.18782/2320-70512721
Abd El-Rahman AF, Shaheen HA, Abd El-Aziz RM et al (2019) Influence of hydrogen cyanide-producing rhizobacteria in controlling the crown gall and root-knot nematode, Meloidogyne incognita. Egypt J Biol Pest Control 29:41. https://doi.org/10.1186/s41938-019-0143-7
Sehrawat A, Sindhu SS, Bernard R, Glick BR (2022) Hydrogen cyanide production by soil bacteria: biological control of pests and promotion of plant growth in sustainable agriculture. Pedosphere 32(1):15–38. https://doi.org/10.1016/S1002-0160(21)60058-9
Sudha A, Durgadevi D, Archana S, Muthukumar A, Suthin RT, Nakkeeran S, Poczai P, Nasif O, Ansari MJ, Sayyed RZ (2022) Unraveling the tripartite interaction of volatile compounds of Streptomyces rochei with grain mold pathogens infecting sorghum. Front in Microbiol 13:923360. https://doi.org/10.3389/fmicb2022923360
Sayyed RZ, Patel PR (2011) Biocontrol potential of siderophore-based heavy metal resistant Alcaligenes sp and Acinetobacter sp vis-à-vis organophosphorus fungicide. Indian J of Microbiol 51(3):266–272. https://doi.org/10.1007/s12088-011-0170-x
Punja ZK, Rodriguez G, Tirajoh A (2016) Effects of Bacillus subtilis strain QST and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot 84:98–104. https://doi.org/10.1016/jcropro201602011
Ahmed S, Choudhury AR, Kumar S, Choi RJ, Sayyed RZ, Sa TM (2021) Biomolecular painstaking utilization and assimilation of phosphorus under indigent stage in agricultural crops. In: Singh HB, Vaishnav A, Sayyed RZ (Eds) Antioxidants in Plant-Microbe Interaction. Springer, Singapore, pp 565–588. https://doi.org/10.1007/978-981-16-1350-0_26
Khan N, Ali S, Shahi MA, Mustafa A, Sayyed RZ, Curaá JA (2021) Insights into the interactions among roots, rhizosphere, and rhizobacteria for improving plant growth and tolerance to abiotic stresses: A review. Cells 10(6):1551. https://doi.org/10.3390/cells10061551
Sayyed RZ, Sonia Seifi, PR Patel, SS Shaikh, HP Jadhav,& Hesham El Enshasy (2019) Siderophore production in groundnut rhizosphere isolates, Achromobacter sp. RZS2 influenced by physicochemical factors and metal ions. Environmental Sustainability Springer. 2;2:117–24. https://doi.org/10.1007/s42398-019-00070-4
Kapadia C, Sayyed RZ, Enshasy HEE, Vaidya H, Sharma D, Patel V, Malek RA, Syed A, Elgorban AM (2021) Ahmad K and Zuan ATK [2021] Halotolerant microbial consortia for sustainable mitigation of salinity stress, growth promotion, and mineral uptake in tomato plant and soil nutrient enrichment. Sustainability 13:8369. https://doi.org/10.3390/su13158369
Rai S, Omar AF, Rehan M, Al-Turki A, Sagar A, Ilyas N, Sayyed RZ, Mirza H (2022) Crop microbiome: their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 257:27. https://doi.org/10.1007/s00425-022-04052-5
Naz H, Sayyed RZ, Khan RU, Wani OA, Maqsood A, Maqsood S, Fahad A, Lim HR, Show PL (2023) Mesorhizobium improves chickpea growth under chromium stress and alleviates chromium contamination of soil. J Environ Manag 338:117779. https://doi.org/10.1016/j.jenvman.2023.117779
Vafa N, Sohrabi Y, Sayyed RZ, Suriani NL, and Datta R (2021)S Effects of combinations of Rhizobacteria, mycorrhizae, and seaweeds on growth and yields in wheat cultivars under the influence of supplementary irrigation Plants, plants 10:81. https://doi.org/10.3390/plants10040811
Acknowledgements
The authors sincerely thank the ICAR-National Institute for Plant Biotechnology, New Delhi, India, for providing the facilities. The authors would like to acknowledge the support provided by Researchers Supporting Project Number RSP2024R358, King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by Università degli Studi di Brescia within the CRUI-CARE Agreement. This work was funded by Researchers Supporting Project Number RSP2024R358, King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Conceptualization and supervision: AS and RZS; writing: AS, SR, and SS; writing—review and editing: RZS, PP, NAB, and KP; formal analysis: AS and SR; fund acquisition: NAB and KP. All authors contributed to editorial changes in the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Informed Consent
Not applicable.
Institutional Review Board Statement
Not applicable.
Competing Interests
The authors declare no competing interests.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sagar, A., Rai, S., Sharma, S. et al. Molecular Characterization Reveals Biodiversity and Biopotential of Rhizobacterial Isolates of Bacillus Spp. Microb Ecol 87, 83 (2024). https://doi.org/10.1007/s00248-024-02397-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00248-024-02397-w